Globally, in 2018, 79% of these people living with HIV (PLWH) knew their status, 78% of whom were receiving treatment and 86% of the treatment group (i.e., 53% of all PLWH) had achieved viral suppression.
This means successful drug regimens were enabling 37.9 million people to live with HIV or AIDS, with an estimated 23.3 million of these individuals having access to treatment. In addition, the approximately 770,000 deaths due to HIV/AIDS recorded in 2018 represented the lowest number of deaths from this disease since 1998.1
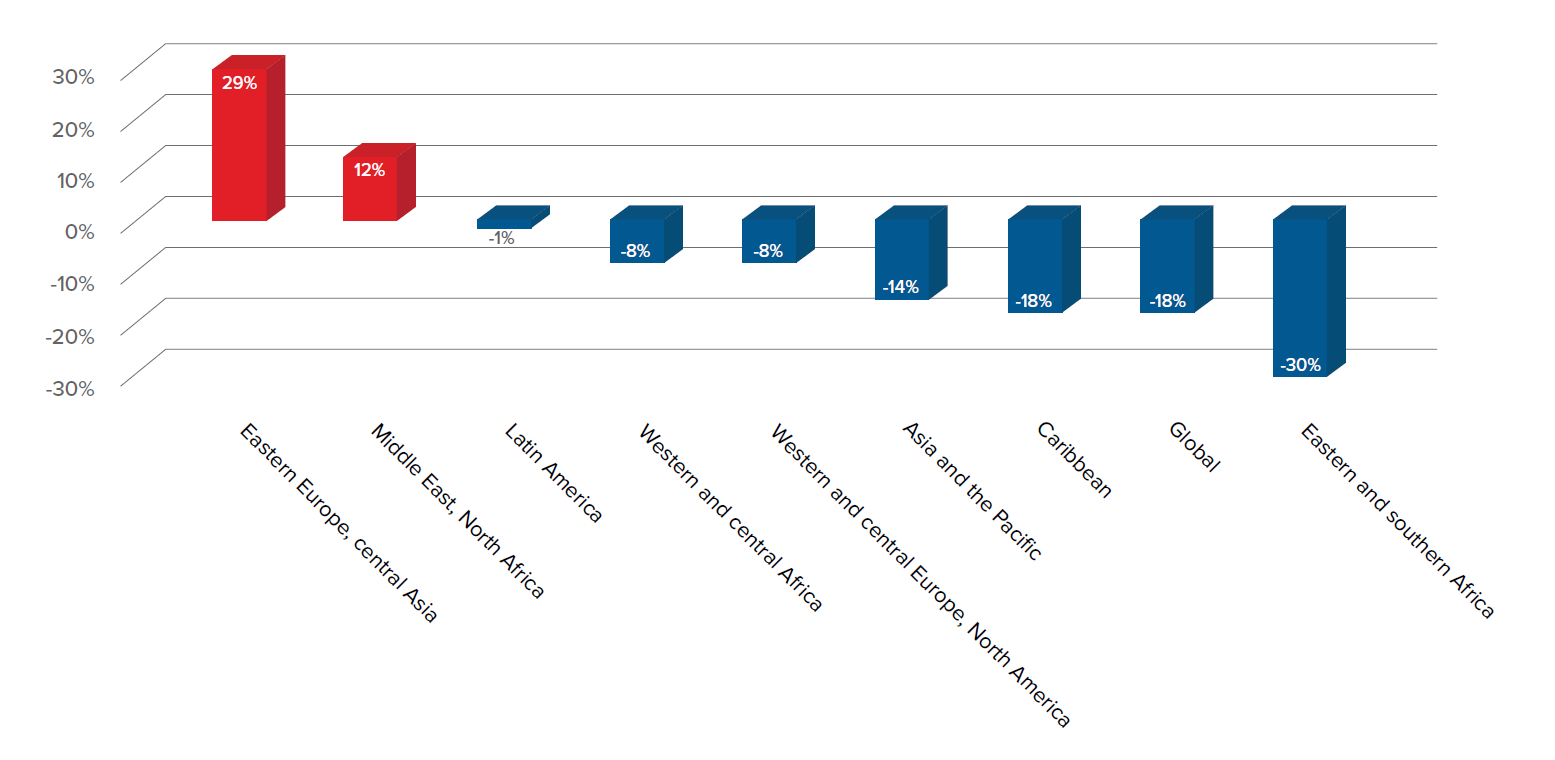
Although incidence has begun falling in many sub-Saharan African countries, the annual number of new HIV infections in Eastern Europe and central Asia has nearly doubled since 2000, and there has been no reduction in mortality from HIV/AIDS in these regions since 2010.2 In addition, transgender people are considered to be one of the groups most affected by HIV/AIDS, with global prevalence as of 2013 ranging from 8% to 68%.3 Key scientific advances in recent years have helped to improve longevity and quality of life, and improve levels of viral control, but until such time as a cure is found, researchers continue to seek out more effective ways to control the disease.
Antiretroviral therapy (ART)
In March 2019, the U.S. Centers for Disease Control (CDC) stated that 80% of new 2016 HIV infections in the U.S. were transmitted by people who either did not know they were HIV-positive, or knew they carried the virus but were not receiving treatment.4
Figure 1:
From 2005 to 2015 the number of people living with HIV or AIDS who were on antiretroviral treatment (ART) rose significantly, from 6.4% to 38.6% of men diagnosed with HIV and from 3.3% to 42.4% of women. However, the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates that up to half of those diagnosed with HIV are not receiving ART. In addition, of those who are, less than half remain virally suppressed three years after starting treatment.3, 6
New drugs with greater barriers to resistance are helping extend the lives of HIV patients. Adherence and effectiveness have been greatly improved by regimens requiring only a single daily tablet. Currently, new dosing methods for ART drugs, such as monthly injections and implants, are under investigation.3, 7
Guidelines for use of ART in PLWH
The Strategic Timing of Antiretroviral Treatment (START) clinical trial, launched in 2009, has illustrated the benefits of starting ART as soon as a patient is diagnosed with HIV rather than waiting for the patient’s CD4 T-cell count to fall below 300 cells/mm3. The risk of AIDS-related conditions and chronic illness and death was 53% lower for those with HIV who started treatment when their CD4 T-cell counts were at least 500 cells/mm3 compared to those who waited until their CD4 count had dropped to 350 cells/mm3 or lower. It is now recommended by WHO that all patients newly diagnosed with HIV be immediately started on treatment, regardless of their CD4 count and viral load.
Regular testing and annual monitoring of viral load in blood is now considered to provide a better assessment of treatment efficacy and viral suppression. Per the World Health Organization (WHO), “Viral load is recommended as the preferred monitoring approach to diagnose and confirm treatment failure.” However, if viral load testing is not routinely available, WHO recommends that CD4 count and clinical monitoring “be used to diagnose treatment failure, with targeted viral load testing to confirm viral failure where possible.” Monitoring the CD4 count is hence of less consequence and viral load testing is now the key focus in assessing viral suppression.8, 9
Once viral load falls below 50 copies/mL, monitoring is recommended every three months until suppression has continued for at least a year. Thereafter, viral load monitoring can be carried out every six months. WHO recommends monitoring patient CD4 counts every six months until it remains above 250 cells/mm3 for at least a year with viral suppression.10
New treatment developments
As longevity improves for PLWH, the risk increases that prolonged exposure to a number of the drugs used to treat it can lead to increased resistance to those drugs. The presence of toxicities in older ART drugs may also prompt a change of regimen. Fortunately, many new HIV drugs have been introduced in recent years that are enabling virally suppressed patients to switch to new and simpler regimens which are easier to manage.
Consolidated and updated WHO guidelines for antiretroviral drugs in the treatment and prevention of HIV recommend two drugs, dolutegravir (DTG) and efavirenz 400 mg (EFV400), as first-line treatment for HIV. EFV400, a lower-dose version of the older efavirenz (EFV600), has demonstrated fewer drug reactions (38%) versus EFV600 (48%). Both of these drugs have been shown to be more effective and better tolerated, with fewer drug interactions and higher genetic barriers to resistance, than older drugs. However, a higher rate of neural tube defects has been found in babies born to mothers taking a dolutegravir-containing regimen at conception.10
WHO also no longer recommends tenofovir alafenamide fumarate (TAF) due to lack of safety data on pregnant women and likely drug interactions between TAF and rifampicin, a drug used to treat tuberculosis (TB) co-infections.11 Bictegravir (BIC), a new integrase strand transfer inhibitor (INSTI), was approved in February 2018 by the U.S. Food and Drug Administration (FDA) for use as part of a single-tablet regimen (STR). The triple therapy STR, which includes TAF and emtricitabine (FTC), is known as Biktarvy and is the recommended first-line treatment for those newly diagnosed with HIV-1.12
Separately, ibalizumab (IBA), a CD4 post-attachment inhibitor, has recently been approved for use in patients with multi-drug resistant HIV. Patients co-infected with hepatitis C (HCV) should take ART drugs which have been demonstrated to have minimal interaction with HCV treatments, such as DTG/abacavir/lamivudine, DTG/TAF/embricitabine, bictegravir/TAF/emtricitabine, or raltegravir plus TAF/emtricitabine.11
DTG and rilpivirine, which is marketed under the name Juluca, was the first complete dual therapy combination approved for use in 2017. However, it is only indicated for HIV patients who are virally suppressed and have been on a stable regimen for at least six months.12
On April 8, 2019, the dual therapy drug Dovato, which combines DTG and lamivudine, was approved by the FDA for use in adults diagnosed with HIV who had no previous treatment history. It is the first once-daily single tablet for treatment-naïve HIV-1 adults. The European Commission approved Dovato for use this past July as well, and the drug is currently under review by regulatory authorities in Canada, Australia, Switzerland, and South Africa. Several submissions for approval are planned for additional countries.13
Finally, although DTG has a higher genetic barrier to resistance, improved efficacy, and is better tolerated by HIV patients, the issue of neural tube defects in children born to mothers receiving this drug at conception is still of concern.14, 15 Long-acting injectable treatments such as cabotegravir-rilpivirine, which is currently in clinical trials, may provide an alternative to daily tablet regimens in the future. Currently in clinical trials, initial results showed good efficacy at 96 weeks as both a monthly and a bi-monthly injection.7
Life expectancy and survival for PLWH
New drug regimens for HIV have all led to better patient adherence and improved survival. However, up to half of HIV patients smoke, and evidence suggests that HIV patients who smoke may lose more years to smoking than to HIV infection itself. Longer life expectancies for PLWH have encouraged these individuals to take better care of their health (e.g., cease smoking) and improve their ART adherence. Reduced toxicity of newer ART drugs is also helping improve life expectancies for HIV patients, which is now approaching that of the general population.16
For longevity, prevention of cancer is key. Evidence suggests that risk of cancer in HIV patients can be reduced with ART: overall risk of cancer in those who start ART when their CD4 count is greater than 500 cells/mm3 is reduced by nearly two-thirds compared to those who start ART with a count of less than 350 cells/mm.2, 4
Longer lives for PLWH also means a greater likelihood this population might develop a range of non-communicable diseases (NCDs) they previously did not live long enough to have. Now, chronic immune activation due to HIV leaves patients more likely than the general population to experience cardiovascular diseases (e.g., myocardial infarction, stroke), diabetes, renal failure, fractures due to osteoporosis, frailty, cognitive decline, and cancer. Indeed, a 2015 study projected that by 2030, at least 84% of the Dutch HIV population will suffer from at least one NCD in addition to HIV.17
Mortality for PLWH
Studies examining the effects of long-term ART have found no evidence of any impact on mortality based on duration of treatment after the initial period of stabilization. More recently, studies have also found that the initial CD4 count at commencement of therapy may not be a significant factor in predicting mortality risks for those on ART 10 years or more.18 Older age, male sex, intravenous drug use, and starting treatment in earlier decades were all associated with higher mortality rates.19
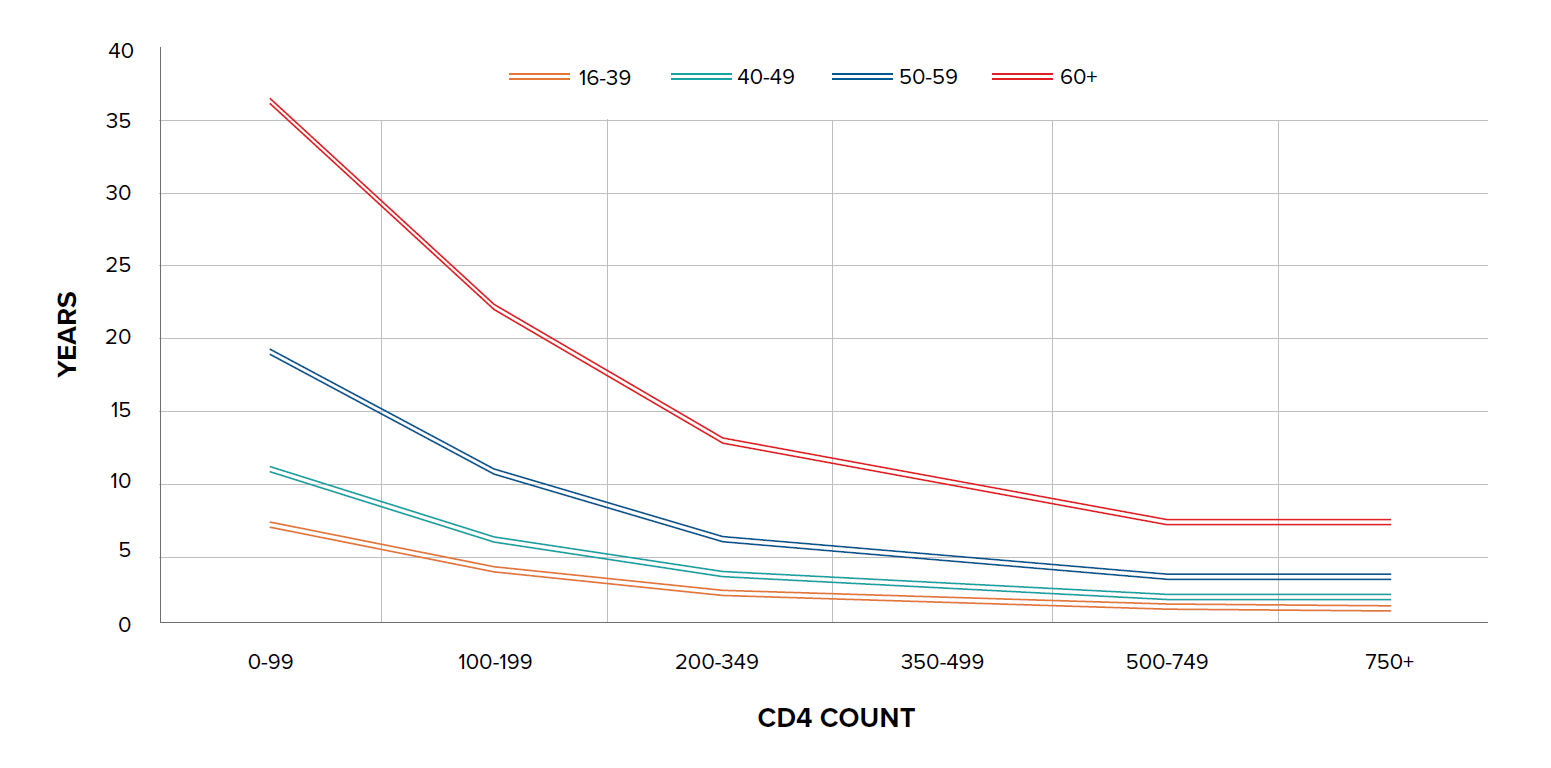
A study in the U.K. found that the estimated life expectancy in 2010 for men who have sex with men (MSM), who do not have hepatitis C and had a high CD4 count at ART initiation, was 75 years – only seven years less than that of the general male U.K. population and similar to that of smokers.20 Another study, by Trickey, et al. on cause-specific mortality in HIV-positive patients from North America and Europe, found that five-year mortality risk was <5% in 60% of all patients and <5% in 30% of those ages 60 and over, with older age being strongly associated with cardiovascular mortality. For those between the ages of 40 and 49 who are 10 years post-starting ART, five-year mortality risk ranged from 1.9% for those not injection drug users (IDUs) who were virally suppressed and had a CD4 count of >500 cells/mm3 and no AIDS, to 48% in those who were IDUs and not virally suppressed with CD4 count <100 cells/mm3![]() and AIDS. In comparison, the mortality risk in the HIV negative population in France was 1%.21
and AIDS. In comparison, the mortality risk in the HIV negative population in France was 1%.21
Figure 2:
Moving forward with HIV treatments
HIV self-testing has encouraged high-risk individuals to monitor their own health and HIV statuses. Promotion of HIV testing has ramped up and home test kits are now available. Implantable sustained-release methods for ART are in development, and long-acting monthly injectable treatments have proven as effective in Phase 3 trials as the most effective once-daily tablet regimen. Indeed, a manufacturer focused on injectables is evaluating the possibility of a long-acting injectable that would be administered every two months.23, 24, 25, 26
Vaccines
Like cancer, HIV today is extremely diverse. A variety of strains now exist that belong to different subtypes, making it difficult to produce a vaccine that would be effective against all strains. The current goal is to produce a vaccine that would cover most major HIV strains. Several vaccine trials are currently under way, with efficacy results expected by 2021 or 2022.
One potential vaccine currently in Phase 1 human clinical trials aims to induce the immune system to produce broadly neutralizing antibodies (bNAbs) which can block HIV from entering healthy cells. While this particular trial is not expected to lead to an effective vaccine, it is hoped that it will lead to a better understanding of how bNAbs impact HIV infection and how to generate these antibodies for a fully protective vaccine. Up to 20% of HIV patients have been found to generate bNAbs against multiple strains of HIV, but this typically only happens two or more years after initial infection.22, 27, 28
New vaccine strategies are also targeting Env, the HIV envelope protein. Env sticks out from the HIV viral membrane in small clusters and adopts very diverse shapes before and after infecting cells. So far, this molecule has been very hard to produce in vaccines. The hope is that scientists can present Env proteins to the immune system as a vaccine in a way that closely resembles the shape of Env in a live virus.
Pre-exposure prophylaxis (PrEP)
One of the most effective ways to prevent HIV transmission from an infected to an uninfected person is for the infected person to attain viral suppression (i.e., HIV-1 RNA <200 copies per mL) through ART.
PrEP is the use of antiretroviral medications in high-risk, HIV-uninfected persons to prevent the acquisition of HIV.
Part 2 of this article will look at the use of PrEP in HIV patients.
A cure?
The recent case of twins born in China in November 2018, following gene editing by Chinese scientist He Jiankui, was focused on editing CCR5 proteins to provide the babies with HIV immunity. However, while one twin had two edited copies of CCR5 allele, the other twin had only one edited copy and one normal CCR5 allele. Neither twin had the CCR5-∆32 mutation, the one found to provide protection from the HIV virus.29, 30
As well as developing improved drug regimens and vaccinations, the search for a cure for HIV continues. Gene therapy is currently being tested to modify host CD4 T-cells to make them resistant to HIV. Anti-cancer immunotherapy is also being tested, as researchers have been able to identify the way in which anti-cancer immunotherapy wakes up the HIV virus and reduces the size of latent HIV reservoirs in people on triple therapy regimens.
In summary
HIV drug regimens today are less toxic, have higher genetic barriers to resistance, are easier to adhere to (one tablet a day), are more effective at suppressing viral load, and have fewer side effects than many of the drugs available 20 years ago. Survival for infected individuals continues to improve as newer, less toxic drugs and simpler one-tablet daily regimens are increasingly available.
There is also greater awareness of how HIV is contracted, and greater availability of disease screening and improved health management, all leading to improved prognoses for people living with HIV. While a vaccine is still proving elusive and a cure yet to be found, science is moving closer each day to ending the HIV epidemic.