Underwriting pulmonary and thyroid nodules has always been a distinct challenge.
Pulmonary nodules are one of the most common incidental findings on a chest X-ray or a computed tomography (CT) scan. Thyroid nodules, on the other hand, are primarily found on ultrasounds, and are presumed to be present in more than half of the global population. In this two-part article we aim to provide information about both types of nodules, which may help simplify their underwriting challenges by identifying factors that can help quantify their risks and provide precise and appropriate guidance.
Part I: Pulmonary Nodules
Background
Pulmonary nodules are characterized as having a focal rounded or irregular opacity, and measuring <3 cm. They can be well or poorly defined, are generally surrounded by lung parenchyma, and are not associated with lung atelectasis (collapse), lymphadenopathy, or pleural effusion.1
A study published in 2015 that tracked incidental pulmonary nodule trends in the U.S. noted that of the 4.8 million study participants who had had a computed tomography (CT) scan between 2006 and 2012, one-third (1.57 million) had an incidental finding of a pulmonary nodule. It was further observed that of these 1.57 million, approximately 4% developed lung cancer within two years of the initial finding.2
Evolution of Solitary Pulmonary Nodule Detection and Diagnostic Capabilities
Chest X-rays, historically, have been the test yielding the most frequent discoveries and diagnoses of incidental pulmonary nodules. The advent of CT scans, however, has led to more precise and accurate diagnoses, as it enables smaller or indistinct pulmonary nodules to be detected and then identified with more precision. The growing global frequency of CT scan utilization has been contributing to a marked increase in incidental pulmonary nodule diagnoses over the last two decades.
Studies indicate that prevalence of malignant pulmonary nodules can vary widely, depending on the means of detection. Initial lung screenings, such as CT scans, yield a rate of incidental nodule findings of between 2% and 24%, whereas for malignant nodules the rate is between 2% and 13%. Advanced tests or follow-up exams such as positron emission tomography (PET) scans result in a much higher discovery rate (46% to 82%).3, 4
Classifying Malignancy Risk
Several frameworks have been developed to classify malignancy risk for pulmonary nodules.
The American College of Chest Physicians (ACCP) guidelines, first published in 2007 and then revised in 2013, utilizes a calculation method developed by investigators at the Mayo Clinic as one of the methods that assesses a nodule’s malignancy risk. It takes into account factors such as age, smoker status, location and diameter of the nodule, presence of spiculation, and history of cancer.4, 5
In 2015, the British Thoracic Society (BTS) published a set of widely used guidelines from which risk calculators were developed to ascertain the malignancy risk of pulmonary nodules.6
The Fleischner Society’s recommendations, which were first published in 2005 and then revised in 2013 and 2017, are today the most widely accepted guidelines for classifying pulmonary nodule risk.7
The Fleischner Society guidelines are based on the following factors:
- Type of nodule
Several types of incidental pulmonary nodules are found on chest X-rays or CT scans. Figure 1 shows the three main pulmonary nodule types – perifissural, solid, and subsolid – and the two types of subsolid nodules (pure ground-glass nodule and part-solid nodule). Each of these types are important to understand and differentiate among, as each has its own bearing on the applicant’s malignancy risk.
- Perifissural nodules (PFNs): These well- circumscribed nodules usually have smooth margins and are found near pulmonary fissures. Generally, no malignancy risk is associated with these nodules.
- Solid nodules: These are the most common type of pulmonary nodules found on chest X-rays or CT scans. These nodules completely obscure the lung parenchyma. They present the most distinct underwriting challenge when assessing their malignancy risk, as the assessment depends on the nodule’s features and associated risk factors.
Figure 1
Classification of Types of Pulmonary Nodules
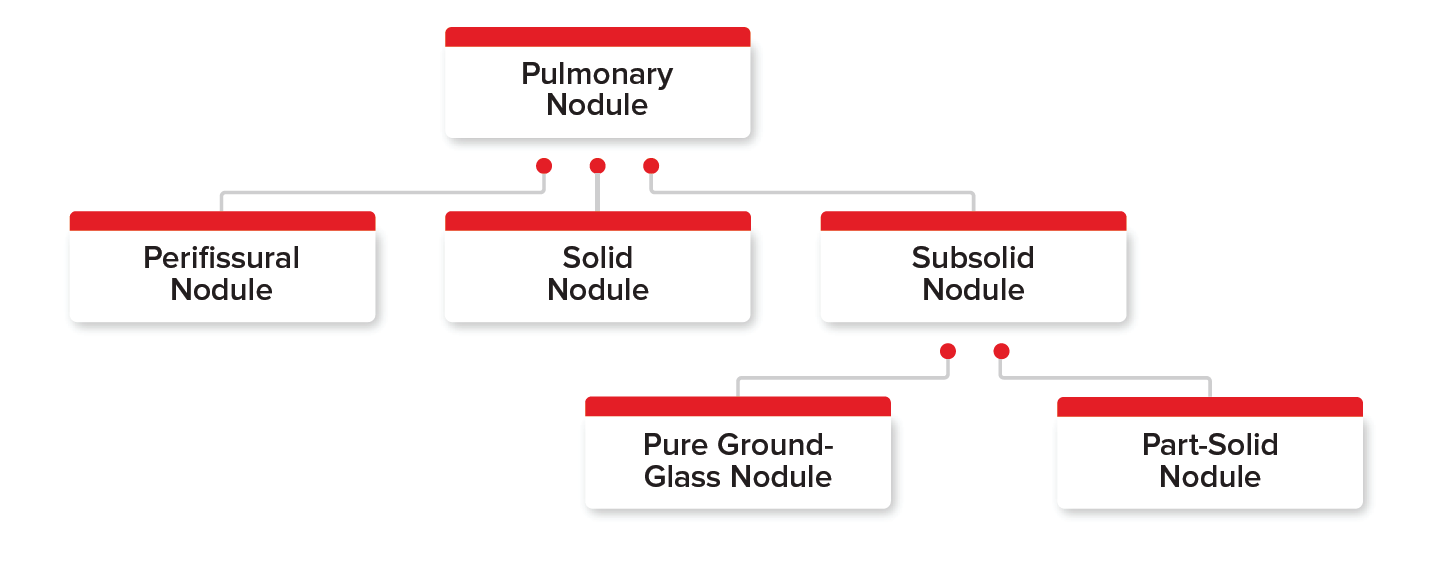
Subsolid nodules: These nodules have two subtypes – pure ground-glass and part-solid. They are found more rarely than solid nodules and both carry a higher malignancy risk than solid nodules (Odds ratio [OR] 1.4).
Pure ground-glass nodules are nodules without opacity that do not obscure bronchial structure or pulmonary vessels. They generally grow slowly in size over a period of many years.
Part-solid nodules contain both ground-glass and solid components. Part of the nodule can completely obscure the lung parenchyma.
Part-solid subsolid nodules have the highest malignancy risk of all nodule types. Their malignancy rate was 63%, whereas the rate for pure ground glass nodules was 18%.3, 6, 7, 8, 9
Nodule size The size of a pulmonary nodule plays an equally important role in determining probability of malignancy, with larger nodules indicating greater malignancy risk.
Prevalence of malignant pulmonary nodules can vary widely, depending on the means of detection.
For example, according to the Fleischner Society guidelines, a solid nodule of less than 6 mm and associated with factors considered to be low-risk (see Table 1, below) is generally benign and needs no further surveillance or follow-up. On the contrary, a solid nodule exceeding 8 mm may represent a poorer prognosis and would require more frequent surveillance, determined by the risk category with which it is associated.7
- Number of nodule(s) found
Multiple pulmonary nodules are also common incidental findings on X-rays or CT scans. Malignancy risk is not necessarily higher if multiple nodules are found. However, closer monitoring may be required in certain cases, based on the “dominant” nodule identified. The dominant nodule is the one that may signify higher risk of malignancy. This may not always be the largest nodule.
The Fleischner guidelines recommend close surveillance of the dominant nodule, especially when different types and sizes constitute the mix of multiple pulmonary nodules. For example, if a solid nodule and a part-solid nodule are found, the risk assessment should consider the part- solid nodule the dominant nodule due to its higher malignancy risk, even if the solid nodule is slightly larger in size.7
- Other risk factors
There are other risk factors which may play a significant role in modifying the malignancy probabilities of a pulmonary nodule. For example, a smaller nodule associated with the high-risk factors listed in Table 1 (below) would need to undergo further surveillance and monitoring, as its malignancy probability is enhanced.7
Table 1
Pulmonary Nodule Risk Factors
| Low-Risk | High-Risk |
|---|
Age* | Younger (age <40 years) | Older (age >50 years) |
|---|
Smoking status | Never smoked OR quit >15 years ago | Current smoker OR quit 15 or fewer years ago |
|---|
Family history of lung cancer | None among first-degree relatives | Present among first-degree relatives |
|---|
Location of the nodule(s) | No upper lobe involvement | Upper lobe involvement |
|---|
Nodule characteristics | Round, smooth margins; presence of cavity | Irregular, spiculated margins; absence of any cavity |
|---|
Personal history of any cancer | No | Yes |
|---|
*Between ages 40 and 50 is considered moderate risk.
Additional Points for Underwriting Consideration
Apart from the primary risk modifiers for pulmonary nodules noted above, the presence of the following secondary risk factors must not be ignored:
selected occupations, e.g., asbestos industry workers or individuals who work with radioactive substances
presence of other pulmonary diseases such as emphysema, infections, or fibrosis
periodic growth of nodule(s) observed on chest X-rays or CT scans on follow-up exams
The presence of these additional risk factors augments the malignancy risk further. Such cases would require complete and detailed up-to-date reports about the nodules for any consideration at the underwriting stage.
On the other hand, a benign nodule often exhibits all or most of the following characteristics:
- diffuse calcification (hamartoma, popcorn calcification), with smooth margins
- absence of any high-risk factors
- no growth of the nodule over a period of two years
- complete absence of any changes in the nodule that would indicate increased malignancy risk
Conclusion
Underwriting pulmonary nodules continues to pose a challenge for underwriters. Although a significant percentage of pulmonary nodules are benign, the fact that lung cancer has one of the highest mortalities among all cancers remains a definitive concern. Thus, a judicious approach to selectively quantify the risk, based on collective features of each nodule and its associated risk factors, is recommended.
Part 2: Thyroid Nodules
Background
Thyroid nodules are solid or fluid-filled masses that form within the thyroid gland. They are one of the most common types of nodule found in the human body, with estimates suggesting that approximately half of the global population is likely to develop a thyroid nodule during their lifetime. The estimated worldwide prevalence is between 19% and 67% on ultrasonography (USG), depending on geographies.10, 11, 12
Over the past few decades, the rapid growth in the use of ultrasound scans globally has led to an exponential increase in the number of incidentally found thyroid nodules, with only 3% to 7% of these nodules discovered upon thyroid palpitation alone.12
There has also been a significant rise in thyroid cancer rates. In the U.S. alone, thyroid cancer incidence increased overall by an alarming 211% between 1975 to 2013, reflecting an average annual incidence increase of 3.6% as well as an annual 1.1% increase in overall mortality rates.13
Risk Classification Methodologies
For underwriters, reports of incidental thyroid nodules found on USGs are common. However, assessing the prognosis of such nodules based on a single USG report has continued to challenge underwriters. A methodical approach to assessing the malignancy risk would be highly beneficial.
Several groups have sought to classify thyroid nodules based on their features. A recent study outlined the differences in relative risk among thyroid nodules by comparing three sets of guidelines: the 2016 guidelines from a consortium of the American Association of Clinical Endocrinologists (AACE), the American College of Endocrinology (ACE), and Associazione Medici Endocrinologi (AME); the 2015 American Thyroid Association (ATA) guidelines for management of thyroid nodules and differentiated cancer; and the 2014 British Thyroid Association (BTA) guidelines for management of thyroid carcinoma.10
The study looked at thyroid nodules based on features found on USGs and categorized them as:
- Low-risk thyroid lesions (benign or low suspicion of malignancy)
- Intermediate-risk thyroid lesions (intermediate or indeterminate suspicion of malignancy)
- High-risk thyroid lesions (high suspicion for malignancy or malignant)
Today, however, the American College of Radiology’s Thyroid Imaging Reporting and Data System, known as ACR TI-RADS (or just TI-RADS), is one of the most accepted thyroid nodule risk classification methodologies.
ACR TI-RADS: A New Chapter for Risk Classification of Thyroid Nodules
Risk classification frameworks for thyroid nodules are not a new development. Several, including Europe’s EU-TIRADS, published by the European Thyroid Association in 2017 and the Korean Society of Thyroid Radiology’s (KSThR) K-TIRADS, published in 2016, assessed malignancy risk using features of the nodules.14, 15
In 2017, the ACR TI-RADS model was introduced, which paved the way for a new classification framework wherein the focus shifted to assigning point values to nodule features and using them to compute a holistic risk score to predict malignancy.16
Table 2 (below) provides a snapshot of the ACR TI-RADS method of risk classification.
Table 2
ACR TI-RADS Scoring System for Thyroid Nodule Classification From USG Images
Composition | Echogenicity | Shape | Margins | Echogenic Foci |
|---|
Cystic or almost cystic | Anechoic | Wider than tall | Smooth | None or large comet-tail artifacts |
Spongiform | Hyperechoic | Taller than wide | Ill-defined | Macrocalcifications |
Mixed cystic and solid | Hypoechoic | | Lobulated or Irregular | Peripheral (rim) calcifications |
Solid or almost completely solid | Very hypoechoic | | Extrathyroidal extension | Punctate echogenic foci |
*Table adapted from ACR TI-RADS, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS committee.16
Each component in the table has been assigned a specific point value, with higher values assigned to more suspicious features. For example, a solid hypoechoic nodule with rim calcifications would have a significantly higher malignancy risk than would a smooth cystic nodule, which is often benign. Thus, each component – solid, hypoechoic, and rim calcification – would have a higher individual point value than would the cystic and smooth components.
The goal is to then calculate a cumulative risk score for each nodule, based on the presence of characteristics indicating a specific TI-RADS category, thereby enabling a more precise assessment of a nodule’s potential malignancy risk. TI-RADS categories can range from 0 to 5, with 0 signifying a benign nodule and 5 representing highest risk of malignancy. For example, a cumulative risk score of 2 would indicate TI-RADS 2, a relatively benign prognosis, whereas a cumulative risk score of 7 or more would indicate TI-RADS 5, a more malignant pathology.
Fine Needle Aspiration’s Role in Thyroid Nodule Diagnostics
The 2017 Bethesda System for Reporting Thyroid Cytopathology, a revision of the system first introduced in 2007, has been universally adopted as the system for classifying thyroid nodules following Fine Needle Aspiration (FNA) evaluation.
Table 3 (below) shows the different diagnostic categories of FNA results and illustrates the corresponding malignancy risk with usual management strategies.17
Table 3
Bethesda System for Classifying Thyroid Nodules Using FNA Results
Bethesda class | Diagnostic category | Malignancy risk (%) | Usual management |
|---|
I | Non-diagnostic or unsatisfactory | 0% - 5% | Repeat FNA with ultrasound guidance |
II | Benign (e.g., benign follicular nodule) | 0% - 3% | Clinical and sonographic follow-up |
III | Atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS) | 10% - 30% | Repeat FNA, molecular testing, or lobectomy |
IV | Follicular neoplasm (or suspicious for follicular neoplasm) | 25% - 40% | Molecular testing, lobectomy |
V | Suspicious for malignancy | 50% - 75% | Near total thyroidectomy or lobectomy |
VI | Malignant | 97% - 99% | Near total thyroidectomy |
*adapted from 2017 Bethesda System for reporting Thyroid Cytopathology: Cibas A, et al.17
It must be noted that there are few predefined criteria for carrying out the FNA tests. Clinicians generally rely on a combination of the TI-RADS score and the size of the nodule to determine if an FNA is warranted. For example, an FNA may be recommend for an EU-TIRADS 5 category nodule when its size is greater than or equal to 1 cm. However, an EU-TIRADS 4 nodule might be considered for FNA if the nodule is 1.5 cm or larger, and an EU-TIRADS 3 nodule considered for FNA if it is 2 cm or larger. In cases where multiple nodules are found, FNA is recommended for not more than three nodules, according to risk and size criteria.14
Risk Modifiers
The factors listed below can be used in conjunction with both ACR TI-RADS scores and FNA results, when available, to determine the prognosis of the nodule. The presence of any of these factors may lead to a poorer prognosis, indicating a prudent underwriting approach.10
- Age at onset, particularly adolescence or older ages (age >70 years)
- Presence of first-degree family history of medullary thyroid carcinoma, multiple endocrine neoplasia type 2, or papillary thyroid carcinoma
- Periodic growth of nodule(s), i.e., nodule growth observed in follow-up USG or PET scans
- Associated symptoms such as persistent dysphonia, dysphagia, or dyspnea
- A personal history of head and neck irradiation, particularly during childhood and/or adolescence
- Presence of cervical adenopathy
There remains an ongoing debate whether intranodular vascularity is determinant of malignant potential of a thyroid nodule. A 2017 U.S. study based on more than 20 years of follow-up of 698 individuals noted that intranodular hypervascularity is associated with adenoma/adenomatous (benign) thyroid nodules, whereas a lack of vascularity was found to be indicative of thyroid carcinomas.18
Another study, conducted on 1,024 hospitalized patients in South Korea, found that vascularity discovered upon ultrasonography, in isolation or in combination with gray- scale ultrasound features, was not useful in predicting thyroid cancer. Intranodular vascularity was present in 31% of the individuals with benign nodules compared with 17% with malignant nodules.19
From these studies, it appears that utilization of intranodular vascularity as a factor to predict malignant thyroid nodules may not be always accurate, and it remains controversial. Further studies are warranted to explore this aspect.
Role of Other Tests in Evaluating Thyroid Nodules
The diagnostic process for thyroid nodules can frequently involve additional tests. Here are a few, and their implications.
Thyroid function tests (TSH, T4) are commonly carried out during investigations of thyroid nodule(s). A hyperfunctioning nodule is less likely to be malignant.
Blood thyroglobulin tests are not clinically mandated for the evaluation of thyroid nodules, as their reliability to predict malignancy remains controversial. In fact, it has been observed that in few cases, thyroglobulin was found to be elevated for eventual benign nodules.
The presence of calcitonin, which is produced by parafollicular (C) cells, is considered more reliable as a serum marker for medullary thyroid cancer but may not necessarily play a role in the initial clinical evaluation of a thyroid nodule.20
A diagnosis of indeterminate thyroid lesions presents an ongoing challenge for most radiologists and cytologists. The evaluation of morphological features alone is not always adequate. The application of ancillary molecular testing for indeterminate thyroid FNA specimens has provided better stratification and triage for some of these instances. Research continues to further refine and improve molecular tests, making them more accurate and less expensive, anticipating that they could provide the basis to solve some of the challenges surrounding these types of nodules.21
Conclusion
Thyroid nodules are one of the most common incidental findings seen at underwriting. Although the features of a nodule play a significant role in determining its malignancy risk, it is important not to underestimate associated risk factors, as they also serve as potential risk factors for malignancy. Thoughtful guidance supported by research-based underwriting tools would help an underwriter classify and assess a nodule’s malignancy risk and yield prudent decision-making.