Extended life expectancy has led to many more people living with chronic diseases, who may ultimately require organ transplantation in the treatment of their condition, yet these organs are in short supply.
Xenotransplantation may represent one solution. The possibility of using pig organs in humans could improve mortality rates for conditions such as cardiomyopathy, chronic obstructive pulmonary disease, diabetes, liver cirrhosis and polycystic kidney disease to name but a few.
Xenotransplantion is the transplantation of organs, cells, or tissues between species, for example from pigs to non-human primates (NHPs) or humans. The World Health Organization (WHO) defines xenotransplantation as ‘any procedure that involves the transplantation, implantation or infusion into a human recipient of either (i) live cells, tissues, or organs from a non-human animal source; or (ii) human body fluids, cells, tissues or organs that have had ex vivo contact with live non-human animal cells, tissues or organs’.1
Increasingly, clinicians are turning to this emerging science amid a shortage of organs available for transplant. Recent advances in gene editing and immunosuppressive therapy have meant that xenotransplantation may soon be a viable option to bridge this gap and reduce transplant waiting lists.
Human-to-Human Transplantation History
The first documented human kidney transplant was completed in 1933 in the Soviet Union, but the transplanted kidney never produced urine and the patient died two days later. It was not until 1954 that the first successful human kidney transplant was completed between identical twins, with the recipient surviving eight years with normal graft function. This was followed in 1963 by the first human lung transplant, and by the first human heart transplant in 1967 by Dr Christiaan Barnard. Yet immunosuppression remained a problem and organ grafts were frequently rejected. More recently, face, uterus, and hand transplants have been completed with some success. With the help of immunosuppressive drugs, such as tacrolimus, sirolimis, mycophenolic acid, and everolimus, rejection rates of transplanted human organs can be reduced.2
Advent of Xenotransplantation
Xenotransplantation is not new, with early attempts dating back to the 19th century. Further attempts were made in the 1960s, when an adult female received a chimpanzee kidney and reportedly survived for nine months. The first heart xenotransplant was carried out in 1964, but the patient died two hours later. Heart xenotransplants were again attempted in two patients in 1977 with no success, and in 1984, a neonate infant with hypoplastic left-heart syndrome received a baboon heart and survived for 20 days. Reports also show that in 1992 a patient lived for 70 days after having received a chimpanzee liver. However, these grafts were generally unsuccessful due to acute organ rejection.3, 4
Recently the severe shortage of human organs available for transplantation, along with advances in genetic engineering, have renewed interest in developing pig organs suitable for human transplantation. But why pigs? Pigs mature rapidly, can have numerous litters with multiple offspring, have organs that are relatively similar in size and physiologic capacity as humans, and produce insulin that is biologically active in humans.
Pig breeding is well-established and cost-effective, and the varied breeds allow for organ size to be better matched to a recipient. However, because of differences in genetics, modifications must be made before pig organs can be successfully transplanted into humans. Advances in genetic engineering, such as clustered regularly interspaced short palindromic repeats (CRISPR), have enabled gene editing to take place in vitro to prevent organ rejection. Pigs can now be bred in a sterile environment and are selected for breeding if negative for porcine viruses, bacteria, and fungi.5
Pig Xenotransplantation – A Deeper Dive
In September 2021, a team at New York Langone Medical Center in the US successfully transplanted a genetically engineered pig kidney into a deceased donor who was maintained on a ventilator. The xenograft was able to produce urine for 54 hours without showing any signs of acute rejection before the study was terminated. Yet, there remain two main issues with xenotransplantation. The first is the risk of zoonoses (diseases caused by germs or viruses spread between animals and people), and the second is immunological and hematological risk, leading to organ rejection. While much progress has been made in recent years, barriers remain in combating organ rejection.6
When a xenograft is transplanted into a human, the graft is often quickly destroyed due to hyperacute rejection (HAR). HAR is initiated by antibodies that naturally exist in the recipient, leading to the destruction of the graft vasculature and organ failure. Acute rejection is demonstrated by interstitial hemorrhage, infarction, necrosis, thrombosis, and loss of tubules (found in various organs to transfer fluids).1
To control organ rejection, multiple genes must be first altered in donor pigs. (See Figure 1.) Thus far, up to seven genetic alterations have been made, but it remains unknown if manipulating so many genes in donor pigs will have any long-term adverse health effects for organ recipients. The third WHO Global Consultation on Regulatory Requirements for Xenotransplantation Clinical Trials indicates that donor pigs should have a minimum number of necessary genetic modifications, although the number and combination of modifications have yet to be defined.1, 3 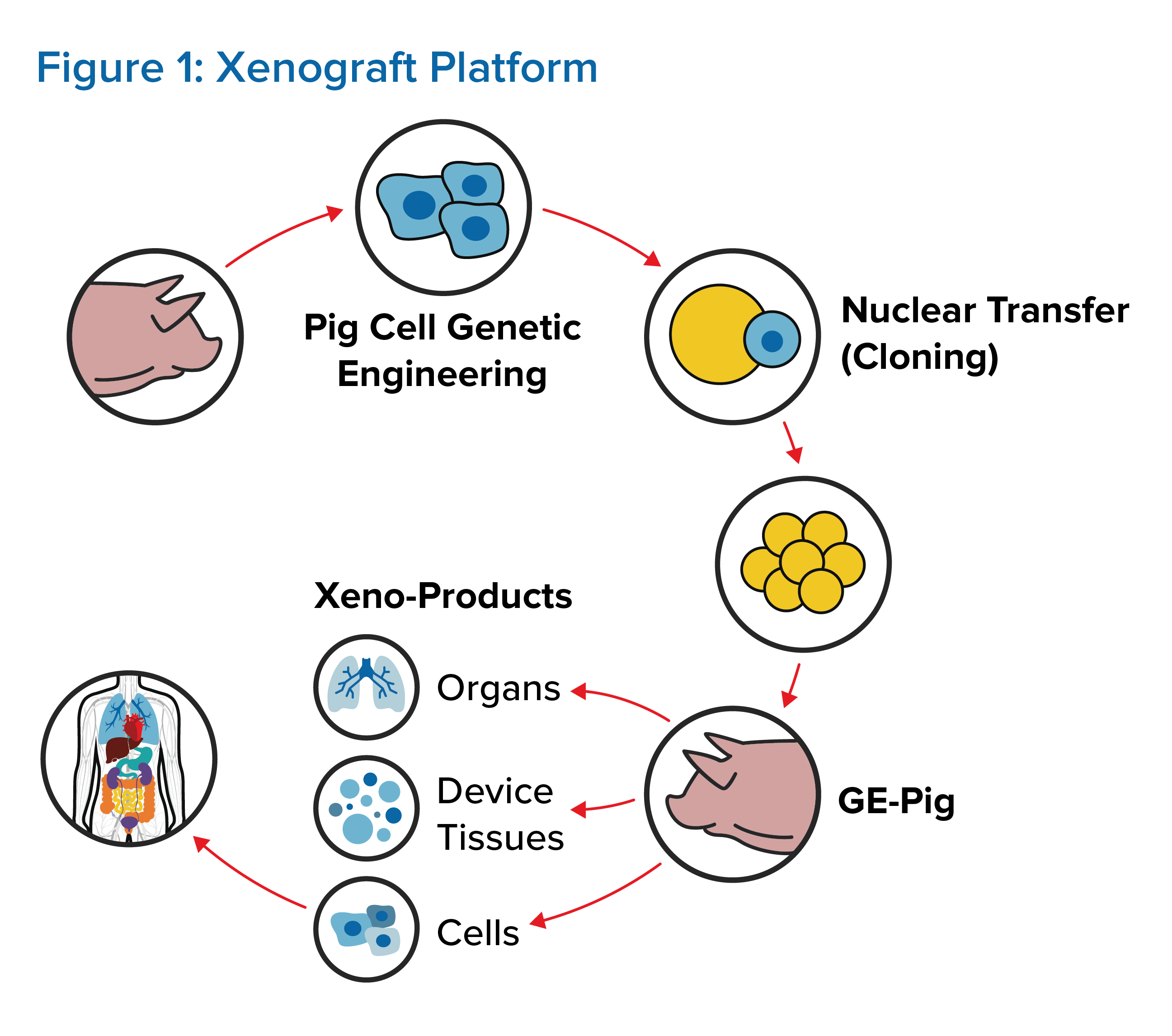
Genetically engineered pigs edited to express the human CD47 protein can potentially help reduce the problem of organ rejection. This, combined with drugs that target coagulation and inflammation, has significantly increased duration of organ function in ex vivo models.
It has also included deleting the antigen galactose-α-1,3-galactose (alpha gal) by GGTA1 gene knockout. Alpha gal is a carbohydrate found in most mammal cell membranes, but some primates and all humans lost the GGTA1 gene during evolution. Hence humans identify alpha gal as foreign and produce antibodies to destroy it, leading to organ rejection following transplantation. Scientists also added a human gene to the pigs to produce a protein called CD46 to slow the organ recipient’s immune response. ’GalSafe’ pigs were approved by the FDA in December 2020 as a source of medicines and food.7
A further problem associated with xenotransplantation is the risk of zoonoses. Some animal bacteria, viruses, and fungi may be harmless in an animal host but prove extremely dangerous to humans. The porcine endogenous retrovirus (PERV) in pigs is the most studied to date. PERV is an integral part of the porcine genome but is dormant in the host. If PERV is present in the xenograft, it can be activated in the human recipient and cause death.
PERV is divided into three types: PERV-A, PERV-B and PERV-C. The first two types are found in all pig breeds but PERV-C is only found in some types. PERV-A and PERV-B can infect human cells in vitro but PERV-C, while inactive in human cells by itself, can display increased infection in human cells in conjunction with PERV-A. The International Xenotransplant Association (IXA) recommends that donor pigs be free of PERV-C for this reason. Using CRISPR gene editing technology, researchers designed two RNA molecules to inactivate 62 copies of the pol gene required for PERV activation. In 2017, the first PERV-free pig was born.1, 5
Recent findings in pig-to-NHP organ transplants demonstrated survival times of more than 900 days for heart xenotransplants, more than 400 days for kidney xenotransplants, and more than 600 days for pancreatic islet xenotransplants. Successful trials have also been completed using pig pancreatic islet cells in human patients with type 1 diabetes, demonstrating a HbA1c of <7% for >600 days with a significant reduction of the frequency of hypoglycemic events. Liver transplants have only survived for 25-29 days, primarily due to the development of lethal coagulation. The pig lung has been found to be the organ most severely damaged by rapid onset coagulation dysfunction.1, 7
The Future
While xenotransplantation has been studied for many years, the European Consortium, which generates data to progress xenotransplantation toward human clinical trials, is also examining ways to use pig neuronal cell transplantation to cure Parkinson’s disease. Research is also underway into such breakthroughs as bioengineered livers and 3D organ printing, an emerging concept in tissue engineering. As the organ is printed, it is also impregnated with human cells, creating a collagen scaffold for use in constructing functional tissue and organs in vitro. However, clinical trials have not yet begun in humans.1, 3
Conclusion
The number of human organs available for transplantation is nowhere near sufficient to meet the ever-increasing demand by patients worldwide. Xenotransplantation has the potential to increase organ supply and reduce waitlist mortality for critically ill people who require organ transplants. However, the risk of zoonotic viral transmission to humans and acute and chronic rejection remains. It is hoped that improvements in gene editing technology may lead to successful genetic manipulation in pigs, removing the risk of organ rejection so that the first clinical trials of xenotransplantation in humans can begin, increasing longevity and improving the quality of life in people who suffer from chronic diseases across the globe.




