Treating PCS
Managing PCS is particularly challenging due to the complexity of symptoms and limited evidence for some treatments. Current approaches include:
Non-pharmacological interventions
- Cognitive rehabilitation: Helps improve cognitive deficits in attention, memory, and executive functioning.
- Psychotherapy such as cognitive behavioral therapy (CBT): Provides coping strategies for emotional symptoms like irritability, depression, and anxiety.
Pharmacological treatments
- Antidepressants and anti-anxiety medications: Used symptomatically to manage emotional and psychological symptoms.
- Stimulants: Sometimes prescribed to improve attention and concentration.
Rehabilitation therapies
- Vestibular and vision therapy: Addresses cervical soft tissue damage often associated with mTBI, although evidence of efficacy remains limited.
The Australian Sports Commission’s 2024 “Concussion and Brain Health” position statement highlights a physiotherapy-guided rehabilitation plan, focusing on five key areas – the autonomic system, cervical spine, vestibular function, vision, and cognition – to guide an athlete’s safe return to activity.13,14,15
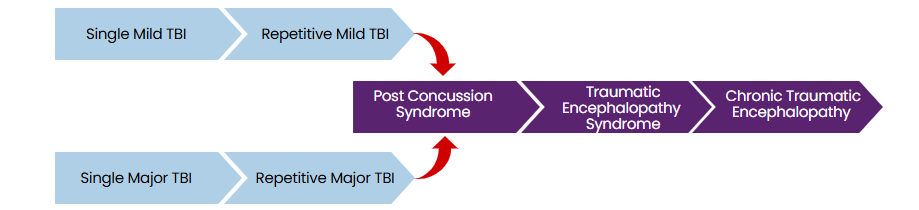
Traumatic encephalopathy syndrome and chronic traumatic encephalopathy
The neuropathological hallmarks of chronic traumatic encephalopathy (CTE-NC) include hyperphosphorylated tau (pTau) protein and neurofibrillary tangles (NFTs) or pretangles in specific brain regions characteristic of the condition. These abnormalities cluster around small blood vessels in the cerebral cortex, leading to progressive and debilitating neurodegeneration.
While tau deposition is common in several neurodegenerative diseases, such as Alzheimer’s, and in normal aging, the pattern and distribution of tau and NFT pathology in CTE are distinct enough to be considered diagnostically definitive.
The mechanisms underlying CTE remain unclear, but repetitive mild traumatic brain injuries (rmTBI) are believed to cause abnormal phosphorylation of tau protein. This misfolded and cleaved protein aggregates into clumps, potentially promoting the accumulation of other aggregate-prone proteins. These pathological changes can be confirmed only through autopsy, as they are not detectable via imaging.
The pathological stages of CTE are illustrated below:
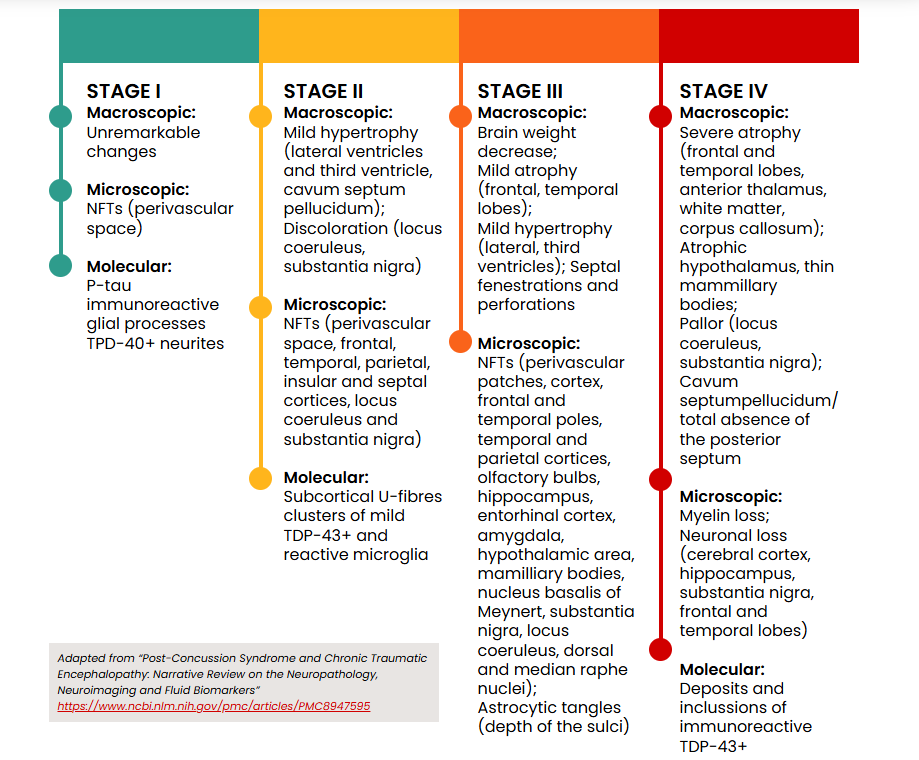
The term CTE-NC (neuropathological changes) refers specifically to the condition diagnosed postmortem. In contrast, traumatic encephalopathy syndrome (TES) describes the clinical signs and symptoms associated with CTE-NC in individuals who are still alive.
Both conditions share features such as impairments in higher executive functioning, including short-term memory loss, mood instability, depression, and difficulties with decision-making and multitasking. TES is also linked to later-life conditions such as dementia and Alzheimer’s disease, and movement disorders like Parkinson’s disease.16
Controversies surrounding CTE
The relationship between repetitive mild traumatic brain injuries (rmTBI) and chronic traumatic encephalopathy (CTE-NC) remains controversial, with ongoing debate over whether rmTBI causes CTE-NC or is correlated with it. CTE-NC is a condition that develops over decades, requiring evaluation of head trauma from the distant past. Each mTBI is typically brief, often ambiguous, and rarely quantified, making rigorous clinical studies difficult.12
Research on CTE-NC largely consists of case reports, retrospective studies, and post-mortem analyses, often with selection bias. Many diagnoses come from retired athletes who donated their brains to sports brain banks.
Environmental factors, such as substance use, genetic predisposition, mental health history, and education level, further complicate causation studies. A 2023 study of 636 cases from the Sydney Brain Bank confirmed a low prevalence of CTE-NC in the general population (0.8%), with only five cases identified -three involving a history of TBI and two without.12
The Concussion in Sport Group’s 2023 “Consensus Statement on Concussion in Sport” acknowledged the potential link between extensive repetitive head impacts, such as those experienced by some professional athletes, and the neuropathology of CTE-NC. This influential group’s concussion protocols are widely adopted across elite and community sports worldwide.17
Investigations for diagnosing concussion
With no specific test or biomarker available, concussion diagnosis and monitoring rely on identifying relevant symptoms and clinical signs.
However, advancements in biomarkers and imaging technologies offer promising new tools for diagnosing mild traumatic brain injuries.
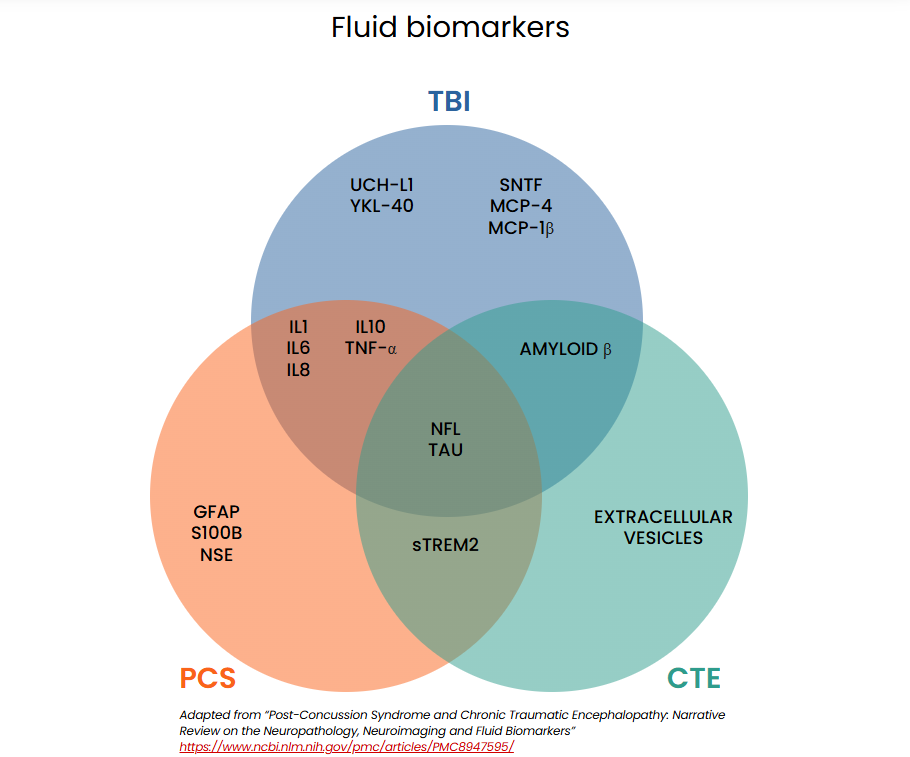
Fluid biomarkers
Brain injuries can cause proteins to leak from damaged cells into cerebrospinal fluid (CSF) or cross the blood-brain barrier into the bloodstream, where they can be measured. This evolving area of research shows promise for diagnosing mTBI. Biomarkers in blood, CSF, saliva, and urine are under investigation to help distinguish concussions from non-concussive head injuries, differentiate mild from severe intracranial injuries, and identify individuals at risk for prolonged symptoms.
The schematic below illustrates biomarkers currently being studied for diagnosing concussion-related conditions.15,18,19
FDA-approved biomarker testing
In February 2018, the US Food and Drug Administration (FDA) approved the first blood test for detecting intracranial injury following a mild traumatic brain injury. This test measures two proteins: ubiquitin C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP), markers of neuronal and glial cell damage. Because each protein originates from different brain cell types, their combined measurement provides complementary insights into intracranial injury. The test was approved specifically for emergency care use in adults over 18 to reduce unnecessary CT scans in patients with mTBI. A negative result within 12 hours of injury is associated with the absence of acute intracranial lesions on CT imaging.
The test is used alongside clinical information, Glasgow Coma Scale (GCS) scores (13-15 for mTBI), and clinical imaging guidelines, such as the Canadian CT Head Rule.21,22
Limitations
- Not validated for individuals under 18 years old
- Detects intracranial bleeding rather than diagnosing concussions specifically
- Approved only for use in emergency settings and within 12 hours of injury
In April 2024, the FDA approved a portable version of the test that delivers lab-quality results within 15 minutes and can evaluate patients up to 24 hours after injury.24 The US Army has partnered with a manufacturer to deploy this proprietary TBI test in field settings, citing nearly 500,000 troops who experienced TBI during training or deployment from 2000 to 2023.25
While these advancements show promise, current biomarkers cannot determine when the brain has fully healed, making them insufficient for guiding return-to-work or return-to-sport decisions. Furthermore, the longer the delay between injury and testing, the less sensitive the results. Continued research into biomarkers holds significant potential for improving concussion diagnostics and care.