What is Robotic Surgery? The simplest definition of robotic surgery (also referred to as robot-assisted or computer-assisted surgery), is this: a human surgeon guiding a robotic unit in the performance of a surgical procedure.
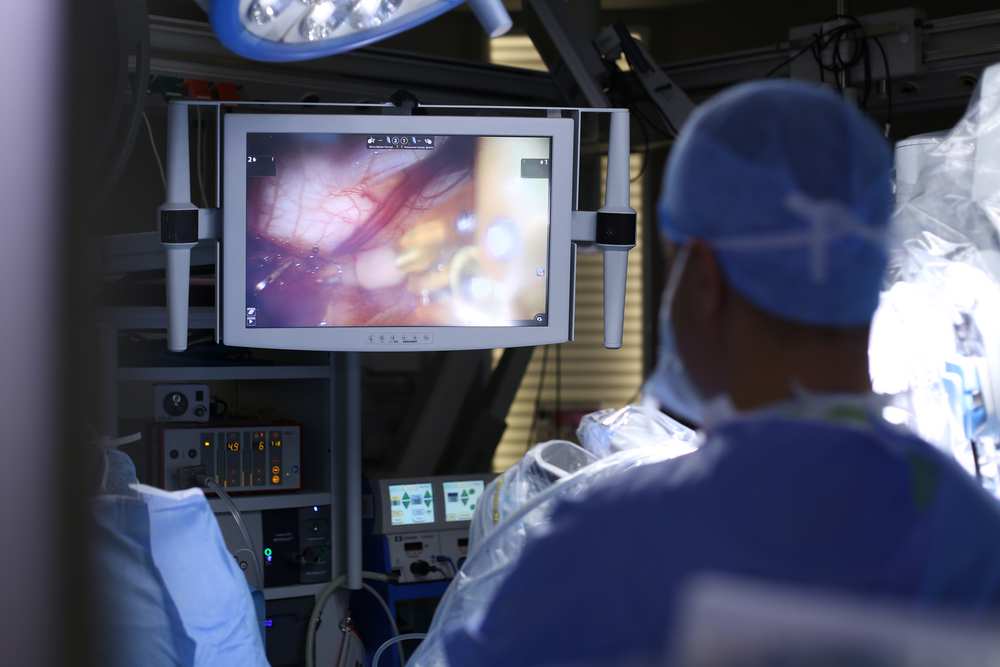
Essentially, a qualified surgeon sits at a console detached from a patient, directing a multi-armed robotic unit in the performance of the actual procedure. A view screen connected to a camera provides a highly magnified three-dimensional view of the surgical site, frequently supplemented by magnetic resonance, ultrasound, or other means of obtaining a three-dimensional perspective.
For surgeons, precision is one of the main advantages of using robots. For laparoscopic procedures, for example, incisions can be far smaller, thereby reducing the possibility of infection and shortening recovery times. For procedures such as open-heart surgery, where brute strength is needed to spread the ribs to open the chest cavity, a robot’s capacity for greater control can make that portion of the procedure far smoother and safer than if it were performed by a human.
For the past several years, a host of procedures have become the province of robot surgical assistance, including laparoscopic prostatectomies, prostate resections, cholecystectomies, appendectomies, hysterectomies, and joint replacements. Indeed, a whopping 86% of the 85,000 prostate surgeries performed in the U.S. in 2009 were robotically assisted. In addition, surgeries involving reconstruction, nerve fiber and blood vessel work, and tumor excision (especially brain tumors) now incorporate robotic assistance, and febotics (or robot-assisted fetal surgery) is a fast-progressing area of current research.
History
The earliest forerunner of a surgical robot – a primitive, programmable mechanical arm that could perform specific tasks – was developed in 1954. Then, in 1975, Victor Scheinman, a graduate student at Stanford University, developed PUMA (Programmable Universal Manipulation Arm), the robotic arm that is now ubiquitous in automobile assembly factories.
The 1980s saw substantial development in surgical robotics. Specialized arms were created for ocular and laparoscopic procedures, and even a surgical scrub nurse robot was built that could respond to voice commands and hand a surgeon his instruments. ARTHROBOT, the first true robotic surgical unit, was also developed in the early 1980s specifically for arthroscopic procedures and was first deployed in Vancouver, Canada in 1983.
By 1985, PUMA had entered the operating theatre. The PUMA 560 robotic surgical arm was used to perform a non-laparoscopic neurosurgical biopsy using computerized tomography guidance, and in 1987, a PUMA surgical arm took part in a laparoscopic gall bladder removal. PROBOT, developed at Imperial College London, relied on preprogrammed movements rather than direction from a surgeon and was used in 1988 to perform a prostatic surgery.
Further robot surgery developments emerged in the 1990s and 2000s. ROBODOC was introduced in 1992 as a system to mill precise fittings in the femur for hip joint replacements. SRI International, with support from NASA and DARPA, developed robotic telesurgery capabilities for zero-gravity and military environments which became the basis for minimally invasive remote on-site surgeries.
Then, in 1999, the da Vinci Surgical System robotic platform was introduced. In 2000, this system became the first to be cleared by the U.S. Food and Drug Administration (FDA) for general laparoscopic surgery. In 2006, a robotic unit conducted a successful human-unassisted (although monitored) surgery to correct heart arrhythmia, and in 2008, the first fully minimally invasive liver resection for living donor transplant was performed. In this surgery, 60% of the liver was removed and the donor left the hospital in just a few days with no complications.
Robot Surgery Today
Specialties that use robotic surgery include urology, gynecology, oncology, otolaryngology, gastrointestinal surgery, pediatric and cardiovascular surgeries, and prostatectomies for prostate cancer.
What robotic surgical units can accomplish in today’s operating rooms can seem nothing short of remarkable. Surgical robot arms can be as small as one centimeter in diameter and can allow precise work down to the microscopic level. Many of the arms are now articulated, providing multiple degrees of freedom (i.e., the number of directions in which they can move), which allows them to mimic more closely the articulated movements of human hands and wrists. Robotic units such as CyberKnife use lasers to enable the radiologic treatment of tumors that might be untreatable via traditional means.
Surgical robot units can also scale a surgeon’s movements so that a large movement at a console can become a very precise dissection or exposure – a benefit for microsurgery procedures. Today’s units can filter out hand tremors, which can be a risk during lengthy and complex procedures. In addition, because a surgeon can remain in a natural, comfortable position during an operation, surgeon fatigue can be reduced.
The number of robotic surgical units currently in use has expanded. By June 2014, 3,102 da Vinci units alone were known to be in use in hospitals worldwide, mostly in the U.S. (2,153), Europe (499), and Japan (183). In addition, at least 150 CyberKnife Systems are known to be installed worldwide.
Still, using these units takes practice and expertise. Surgeons utilizing robots in their practices must have both traditional and robotic surgical competency. Mastery of these systems, depending on the surgery, can take as many as 150 to 200 cases. Also, although surgeons are in complete control of these units, there can be risks – the units can malfunction or stop working entirely. Surgeons must be prepared to switch to hands-on should such a malfunction occur.
Claims Considerations
Although robotic assistance has, for certain surgical procedures, revolutionized the operating theater, there are aspects that need to be taken into consideration by claims assessors, such as:
- Shorter hospitalization / recovery times. Most robot-assisted surgical procedures are minimally invasive. Incisions needed for laparoscopic surgeries
are far smaller, which typically results in reduced infection risk, shorter recovery times, and reduced probability of readmission for complications. When a claims assessor is reviewing the period of hospitalization for a condition, a shorter period might be expected for a robotic than for a traditional surgery, and any extended periods of hospitalization should be reviewed against policy criteria governing medical necessity. - Appropriateness of utilization. Most policies exclude experimental treatment. Claims assessors should ensure that any robotic surgical procedure is performed in accordance with that robotic unit’s specific license. If a unit has been licensed for the performance of a specified procedure, then using it for other procedures may be deemed experimental treatment and possibly excluded.
- Surgeon training and credentialing. Surgeons must already be experts in traditional surgical procedures before utilizing robotic units in the clinical space. The FDA has in place a mandate that requires robot surgery unit manufacturers to provide at least some training. In the case of minimally invasive radical prostatectomies (MIRPs), for example, surgeons must first take a two-day course to learn how to use these surgical units, and then be proctored by surgeons experienced in these robotic-assisted surgeries for at least 20 procedures. Insurers should not be expected to pay for training and should only reimburse the fee of the surgeon undertaking the procedure and not anybody overseeing the operation.
Underwriting Considerations
While full disclosure is necessary and knowledge of the type of procedure undertaken will inform an underwriter’s decision, how the procedure was performed would not normally influence an underwriter’s evaluation of the applicant, as it rightfully should be based on the claimant’s latest health status. An applicant undergoing robot-assisted surgery might benefit from a quicker recovery time following surgery, which may enable an underwriter to accept that applicant sooner than an applicant undergoing traditional surgery, but it will be the applicant’s state of health at the time of application that will be the deciding factor rather than the method of surgery.
Policy Design and Pricing Considerations
Those involved in policy development, benefit design, and pricing should be aware of and consider how the development and growth in the use of robotic surgery will impact policy design and pricing. One item to consider is the high cost of the equipment and therefore surgeries performed using it. Robotic surgical units can cost, on average, US$1.5 million to US$2 million, and the annual servicing contracts can cost an additional US$100,000 to US$170,000. Associated medical fees will also be higher, which will impact pricing. If surgeons’ fees are subject to a benefit limit, these may need to be adjusted to reflect the higher fees.


3b6df65b-f107-43ac-9b32-d8a308104d95.png?sfvrsn=8a342d60_3)