Addictive disorders
One unexpected finding in GLP-1 users has been a reduction in alcohol consumption and addictive behaviors. This may be related to the impact on the brain’s reward system, as GLP-1s are expressed in areas of the brain influenced by addictive drugs, such as nicotine, opioids, and alcohol. The brain’s reward system triggers the release of dopamine, but this response is muted in the presence of drugs such as semaglutide.9
Studies to date have shown that semaglutide, liraglutide, and dulaglutide can reduce alcohol consumption and combat addiction. In one study of over 83,000 patients, semaglutide was associated with a 50%-56% lower risk for both incidence and recurrence of alcohol use disorder over a 12-month follow-up compared with other anti-obesity medications.10
Ongoing trials are currently studying semaglutide and tirzepatide for alcohol use disorder, semaglutide for opioid use and cannabis use disorder, liraglutide for opioid use disorder, and semaglutide and exenatide to achieve smoking cessation.9
Mental health
Diabetic patients treated with tirzepatide, semaglutide, dulaglutide, and exenatide are less likely to be diagnosed with depression and anxiety. A recent study using 233 million patient records found significantly lower depression and anxiety scores for patients that were treated with GLP-1s, particularly with tirzepatide.11
Figure 2: The likelihood of patients being diagnosed with depression after a GLP-1 prescription compared to those on a non-GLP-1 medication11
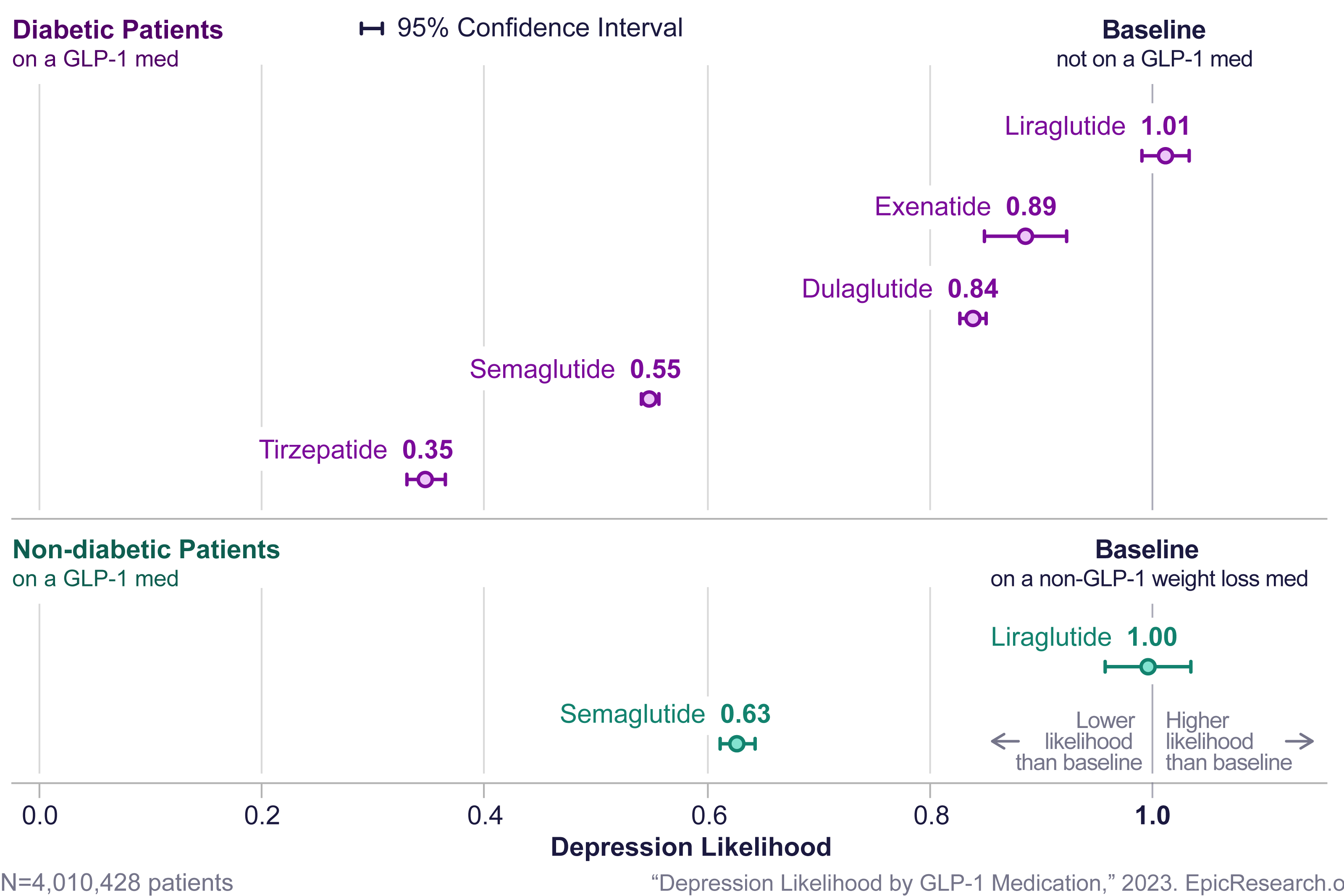
Initial studies on the risk of suicide in individuals taking GLP-1s indicate that these drugs may increase suicidal thoughts and ideation. However, in a retrospective study of nearly half a million patients, semaglutide was associated with lower risk for incident (HR = 0.27) and recurrent (HR = 0.44) suicidal ideation compared with non-GLP-1 anti-obesity medications.12 A separate study of people with T2D treated with GLP-1s found the rate of suicide attempts was more than 53% lower in the GLP-1 group compared to patients treated with DPP-4 inhibitors (oral hypoglycemics).9
Kidney disease
Diabetes is a known risk factor for acute kidney disease (AKD), which is associated with the risk of end-stage kidney disease and mortality. Trials to date have shown that GLP-1s may have beneficial effects on kidney function, especially relevant for T2D patients with chronic kidney disease (CKD).13
- In a recent study of T2D patients with AKD, the mortality rate was significantly lower in the GLP-1 user group compared to the non-user group (HR 0.57). The user group had lower risk of major adverse cardiovascular events (MACE) (HR 0.88), as well as a lower risk of major adverse kidney events (MAKE) (HR 0.73).13
- In the SURPASS-4 trial (phase III), participants taking tirzepatide showed a much lower occurrence of composite kidney endpoint (time to first occurrence of eGFR decline of at least 40% from baseline, end-stage kidney disease, death due to kidney failure, or new onset albuminuria) compared with those who received insulin glargine (HR 0.58).14
- In the Evaluate Renal Function with Semaglutide Once Weekly (FLOW) trial, the risk of a primary outcome event – major kidney disease event, onset of kidney failure (dialysis, transplant, eGFR <15 ml/min per 1.73 m2), < 50% reduction in eGFR from baseline, or death from kidney-related or cardiovascular causes = in patients with T2D and chronic kidney disease was 24% lower in the 1mg semaglutide group compared to the placebo group. The risk of MACE was 18% lower, and the risk of death was 20% lower in the semaglutide group compared to the placebo group.15
Cancers
GLP-1s have been investigated for their impact in reducing the risk of obesity-related cancers (OACs) such as colorectal cancer, esophageal, breast, colorectal, endometrial, gallbladder, stomach, kidney, ovarian, pancreatic, and thyroid cancer, as well as hepatocellular carcinoma, meningioma, and multiple myeloma. In a study of more than 1.6 million patients with T2D and no prior diagnosis of OACs, patients treated with GLP-1s had a significantly lower risk for 10 of the listed cancers.16
Figure 3: Risk of OACs in patients on GLP-1s versus patients on insulin
Conclusion
GLP-1s, initially developed to help manage and treat diabetes and later used to treat obesity, are now showing significant potential to help prevent and treat CVD, mental health disorders, liver and kidney disorders, neurodegenerative disorders, addiction disorders, and cancer. Studies have shown improvements in CVD and all-cause mortality in diabetic patients on GLP-1s. Additional clinical trials are required to evaluate the efficacy, safety, and tolerability of the different GLP-1s for disease management and for reducing cause-specific and all-cause mortality.
This research could have significant implications for the life and health industry. Insurers should carefully monitor these trials and consider GLP-1s’ potential impact on disease incidence, prevalence, and mortality outcomes in the future.
RGA life and health insurance experts are actively engaging with insurers to help determine optimal paths forward in addressing GLP-1 challenges and other emerging issues. Ready to join the conversation? Contact RGA.