The complex anatomic structure of cerebral arteriovenous malformations (AVMs) can present both physicians and underwriters with many challenges, including correct diagnosis and classification, appropriate risk prognostication, and most important, selection of appropriate treatment.
This article clarifies the often confusing nomenclature of cerebral vascular lesions, discusses epidemiology and clinical presentation, and reviews some studies that have significantly altered their treatment paradigms. It also clarifies assessment criteria underwriters can use for risk prognostication and mortality prediction.
Nomenclature of Cerebral Vascular Lesions
Characterizing and risk-classifying cerebral vascular lesions can be quite confusing for underwriters, as radiologists and treating physicians will often use the existing variety of descriptive terms interchangeably and incorrectly. A simplified classification system is presented here in Table 1.
Table 1:
Simplified Classification of Cerebral Vascular Lesions
High-Flow Lesions
- Cerebral AVM (CAVM)
- Dural Arteriovenous Fistula (DAVF) (Can also be low-flow)
- Carotid Cavernous Fistula (CCF) (Can also be low-flow)
Low-Flow Lesions
- Venous
– Developmental Venous Anomalies (DVA)/Venous Angioma
– Vein of Galen Malformation (Can also be high-flow)
- Capillary Telangiectasia
- Cavernous Hemangioma
- Mixed Vascular Lesion
Modified from Radiopaedia.org (https://radiopaedia.org/articles/cerebral-vascular-malformations)
The first step in both risk assessment and classification is to separate high-flow from low-flow lesions. The classic high-flow lesion is an arteriovenous malformation (AVM), a three-part vascular lesion consisting of: a feeding artery (or arteries); a draining vein (or veins); and a nidus, the actual tangle of vessels between the feeding artery and the draining vein. The capillary system normally functions to gradually reduce the pressure gradient from artery to vein, but an AVM causes blood to bypass the capillary system and brain tissue, generating elevated cerebrovascular pressures.
Cerebral AVMs can be graded using the Spetzler-Martin AVM Grading System1 (Table 2). This system takes into account a lesion’s size, the eloquence of adjacent brain tissue (eloquent brain tissue refers to the degree to which that area is responsible for multiple functions), and the amount and type of venous drainage (superficial or deep). Each of these factors have point values, and the sum of the values of these factors results in a grade based upon the sum of the point values, which will be between 1 and 5. A Grade 1 AVM is small and superficial, is located in non-eloquent brain tissue and is considered low-risk for surgery. A Grade 4 or 5 AVM, on the other hand, is large and deep, and adjacent to eloquent brain tissue. (The system uses the term “Grade 6” to refer to an inoperable lesion.)
Although several academic centers have developed treatment protocols corresponding to each Spetzler-Martin grade, it would be inadvisable for an underwriter to draw any conclusions as to mortality outcomes based on these grades alone, as studies to date have been small, retrospective and, most notably, non-randomized to multiple classes of treatment.
Table 2:
Spetzler-Martin AVM Grading System
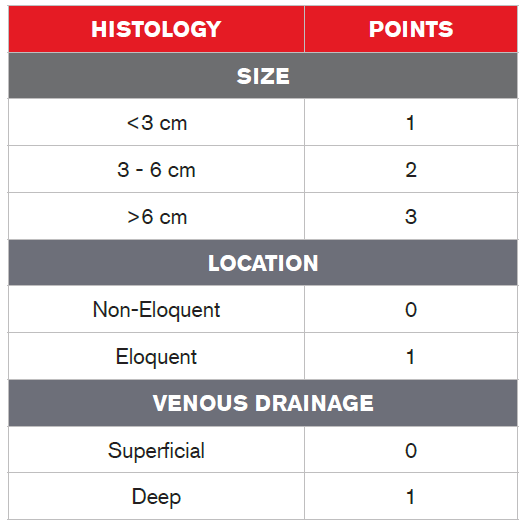
Grade = Total Points
An AVF – arteriovenous fistula – although considered a higher-flow lesion, differs from an AVM in that it presents with a direct connection between a feeding artery and a draining vein and lacks an intervening nidus. The most common location for an AVF occurs between the carotid artery and cavernous sinus, and these lesions are specifically termed carotid-cavernous fistulas (CCF). Another common AVF consists of a direct connection between a meningeal artery and meningeal vein that traverses the dura, and is known as a dural arteriovenous fistula (DAVF).
The most common low-flow lesion is a developmental venous anomaly (DVA). In most classifications schemes, the term DVA has supplanted the older term “venous angioma,” although the latter terminology is still frequently encountered in radiology and physician reports. As the name implies, DVAs are considered congenital lesions. They have larger-than-normal collections of veins, but are for the most part small and histologically normal, and thus represent a low overall bleed risk. They can, however, be frequently associated with the cavernous malformation types described below.
For ease of classification, Table 1 lists the vein of Galen malformation as a low-flow lesion, but these can also exist in high-flow, AVM, AVF, aneurysmal, and other forms as well. Vein of Galen malformations are abnormal connections between the cerebral arteries and the vein of Galen (the main draining deep vein of the brain). Although these lesions are rare, they can be significant: large pediatric vein of Galen malformations, for example, can be the cause of high-output cardiac failure in infants or children, as their hearts struggle to maintain the vascular demands of these lesions.
Cavernous hemangiomas, which are also classified as low-flow AVMs, may have the most names. Nomenclature encountered in physician reports includes cavernomas, cavernous angiomas, and cerebral cavernous malformations (CCMs). This particular lesion type refers specifically to compact collections of abnormally dilated blood vessels without intervening normal brain tissue which are prone to small hemorrhages. Note that these and other vascular lesions are classified histologically by the International Society for the Study of Vascular Anomalies (ISSVA) as “benign tumors,” so for critical illness underwriting purposes it would be beneficial to either specifically include or exclude coverage of these lesions and price accordingly.
Capillary telangiectasias are low-flow lesions that present with a small collection of dilated capillaries. They are distinguished from cavernous hemangiomas by the presence of normally interspersed brain tissue. These are most commonly found in the brain’s pontine region and are usually clinically asymptomatic, and therefore considered lower in risk from an insurance standpoint. These lesions are unrelated to hereditary hemorrhagic telangiectasias.
Mixed vascular lesions have varying combinations of components from the other vascular lesions. These can take several forms, and the insurance risk can vary, depending on the composition of the lesion.
Epidemiology and Clinical Presentation
According to recent studies, the estimated incidence and prevalence of cerebral AVMs is 1 per 100,000 and 18 per 100,000, respectively2. The studies, however, vary greatly in terms of how they define AVMs and in indicating whether the AVMs were symptomatic at diagnosis. When pricing for these lesions, it can be reasonably predicted that overall incidence figures are likely to increase over time due to both increasing utilization of radiographic studies and greater sensitivity of imaging techniques.
Surprisingly, few risk factors for AVMs have been established, but they do exist. AVMs are slightly more common in males. They occur in varying frequencies as part of neurocutaneous disorders such as hereditary hemorrhagic telangiectasia (HHT), Sturge-Weber syndrome (encephelotrigeminal angiomatosis), Wyburn-Mason syndrome, and blue rubber bleb nevus syndrome (BRBNS).
While there is significant variation, AVMs also tend to be a disorder of younger adults. In a large retrospective study, the average age of symptomatic presentation was 36 years. Symptoms can include hemorrhage (46%), seizures (24%), headache (14%), and focal deficit (8%), either singly or in combination. Only 4% of cases were asymptomatic or incidental findings3.
Treatment
The primary clinical consideration upon discovery of an AVM is whether to pursue medical or surgical treatment. Medical treatments commonly include medications to ensure adequate blood pressure control and antiepileptic medications for seizure prophylaxis, although there is little evidence to support either of these interventions. Conservative medical therapy also indicates that patients’ AVMs will be followed with serial imaging studies.
Surgically, an increasing array of options are available. The traditional surgical therapy consisted of resecting the lesion with craniotomy (ligating the feeder arteries and resecting the nidus)4. The decision to pursue an open procedure depended largely on the accessibility of the lesion.
Currently, less invasive surgical options are available. One, embolization of an AVM, involves injection of a thrombosing, embolic, or coiling agent, usually in multiple stages. Embolization can be pursued as definitive therapy, palliative therapy (e.g. to reduce symptoms only), or to reduce the size of the lesion prior to subsequent surgical extirpation or radiosurgery. Radiosurgery (also termed radiotherapy) is another, and involves directing focused radiation to the AVM to elicit a thrombotic effect. Radiosurgery can be pursued in multiple stages, in conjunction with embolization and/or surgical resection.
Today, surgery is performed less often as treatment for these lesions. The 2014 ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations)5 significantly dampened enthusiasm for surgical intervention of these lesions. This trial, the first large-scale randomized prospective study to compare serious outcomes (death or symptomatic hemorrhage) in adults with a history of unruptured AVMs who were treated with medical therapies versus intervention (some combination of surgery, embolization or radiotherapy), found that at a mean of 33 months, the group receiving medical therapy had significantly better outcomes (RR of 0.27 for the combination of death and stroke) than those in the surgical group. Extending analysis of the study group to five years only further supported non-surgical treatments. Additionally, a 12-year prospective study from Scotland7 published near the same time also supported conservative management of these lesions.
Several criticisms have been directed at these studies, including limited follow-up time, selection bias, and failure to standardize the treatment arm. While these studies have had their detractors, it is increasingly clear that consistently viewing untreated AVMs less favorably than treated AVMs from an underwriting risk perspective may not be warranted, especially in the absence of presenting hemorrhage.
Mortality and Risk Prognostication
Unfortunately, large-scale prospective population-based mortality data for cerebral AVMs is not currently available. Survival data varies significantly, based upon populations studied (e.g., admitted to the hospital versus outpatient) and means of presentation (hemorrhage versus other symptoms). One must therefore be careful when applying the data to insured populations. The best available data according to a systematic review of available research2 suggests a case fatality rate of between 1% and 1.5% per year. The same research also offered a first-ever hemorrhage rate of approximately 2% per year. Gross et al.8 reported a recurrent hemorrhage rate of 8% (and of 16% if it occurs within the first year), indicating that the risk of recurrent hemorrhage is higher than of first hemorrhage.
The Spetzler-Martin grading system, meanwhile, implies several factors that have been associated with higher risk of adverse outcome. A deep location, for example, implies both more eloquent brain at risk and greater access challenges. As hemorrhage represents by far the greatest cause-specific mortality risk, prognostic factors from studies9,10 examining both hemorrhage and death can be reasonably combined and summarized (Table 3).
Table 3:
Factors to Consider When Underwriting Cerebral AVM
Favorable
- Asymptomatic/no history of hemorrhage
- Small (<3 cm)
- Female gender
- Superficial location
- Well-defined nidus and >1 draining vein
Conclusion
Perhaps the most challenging aspect of evaluating cerebral AVMs for underwriters as well as clinicians is their heterogeneity. These lesions occur in many diverse patient populations, innumerable anatomic variations, and with no single stereotypical presentation.
The first step for underwriters and clinicians both is to make sure the nomenclature being applied to the lesion is appropriate. The next consideration is whether the lesion has presented with hemorrhage, with another symptom complex, or was found incidentally. Finally, anatomic and individual patient characteristics can be used to refine risk prognostication. Emerging data suggests that conservative treatments may be the preferred approach in many individuals. Developing this stepwise framework to assess cerebral AVMs offers the most effective approach to demystifying this tangled web.