Peritoneal implants
BOT can be confined to the ovary or can be seen in the peritoneum and/or the lymph nodes. Implants in the peritoneum or omentum are not uncommon in serous BOT and may be evident in about a third of BOT patients at surgery.5 The presence of implants is often associated with an exophytic neoplasm (tumor on the surface of the ovary), as fragments of tumor on the surface of the ovary can easily break off and settle in other sites away from the ovary, such as in the pelvis and abdomen.9
Previously, BOT implants were classified as either non-invasive or invasive, depending on whether destructive stromal invasion was identified. According to the 2014 WHO classification, however, the presence of invasive implants represents cancer – specifically, low-grade serous carcinoma (LGSC).3 The classification also defines the two types of non-invasive implants as 1) epithelial, in that it develops in the ovary’s epithelial tissue, or 2) desmoplastic, as it is characterized by nests of epithelial cells within a reactive stroma.
Molecular studies have found that borderline serous tumors and LGSCs carry similar mutations in the BRAF and KRAS oncogenes, implying the existence of a spectrum of change within LGSCs that develop from serous BOT.8
Borderline ovarian tumor (BOT) is an intermediate condition in the tumor spectrum.
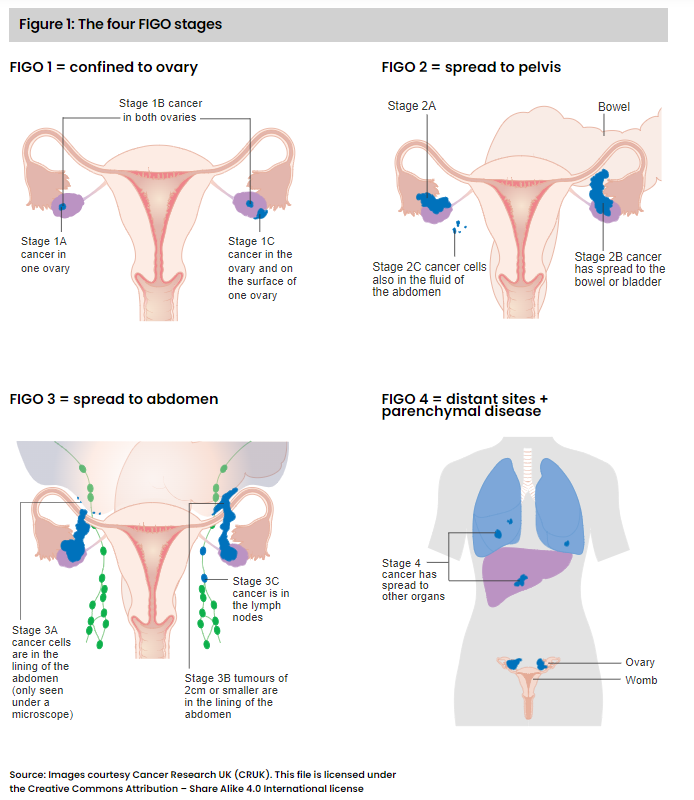
FIGO staging of ovarian tumors
FIGO is a staging system developed by the International Federation of Gynecology and Obstetrics (FIGO) specifically for female cancers, including cervical, ovarian, uterine, endometrial, and BOT. Each FIGO stage (see Figure 1) describes the anatomical extent of tumor presence within the body. However, it does not indicate whether the tumor is an invasive cancer.
Case Study 1: Critical illness claim
A 40-year-old woman presented with abdominal bloating. A CT scan revealed multiple tumor masses in her pelvis and abdomen, and a blood test for presence of the protein CA125, a marker for ovarian tumors, showed a level of 85 units/ml, substantially above the normal range of 0 to 35 units/ml. The woman underwent surgery to remove the tumors, and the surgeon also biopsied suspicious nearby lymph nodes that looked larger than normal.
The histopathology report confirmed borderline serous tumor of both ovaries and the lymph nodes. In addition, non-invasive desmoplastic implants were evident in the peritoneum of the abdomen. The FIGO stage was given as 3B, in view of the spread beyond the pelvis of tumors of up to 2 cm in diameter and the peritoneal implants.
To qualify for a benefit, critical illness policy definitions generally require a diagnosis of malignant tumor with histological confirmation, characterized by uncontrolled growth with invasion and destruction of normal tissue. Diagnoses such as premalignant, non-invasive, carcinoma in situ (Tis) or Ta, borderline malignancy, or low malignant potential, are generally not covered.
In this specific case, there was histological confirmation of a borderline ovarian neoplasm. Also, although there were signs of tumor within the peritoneum with non-invasive implants and presence in the lymph nodes, there was no invasive cancer.
Non-invasive implants are not uncommon and are thought to originate from cell fragments breaking off from the BOT.9 They can generally be seen on the surface of the opposite ovary or other organs within the pelvis and abdomen as well as in the peritoneum, omentum, diaphragm, and abdominal wall.
About one-fourth of women with BOT can also have signs of BOT cells in lymph nodes near the ovaries, but this is not due to invasive cancer and does not appear to influence long-term survival.11
In this case, the claim was deemed to not meet the benefit definition as there was no invasive cancer.
Clinical behavior of BOT
The majority of women presenting with BOT are of childbearing age (premenopausal) and about one-third are under age 40. Most have FIGO stage 1 disease, with tumors in both ovaries in 25% to 50% of cases.12
The mainstay of treatment is surgery, as chemotherapy is generally not effective for BOT. As the majority of women who develop BOT want to preserve their fertility, the surgeon will usually remove only the lesion(s) and not the ovary itself. In the future, if the patient wishes, she might have a second prophylactic surgery to remove the opposite ovary and the uterus. The timing of such a surgery is usually after the woman has completed her family or after menopause.
Life expectancy for these cases is excellent.13 A meta-analysis of 97 trials with more than 4,000 women after a mean follow-up duration of about seven years found that for women with FIGO stage 1 tumors, the overall disease-specific survival rate was 99.5%. For women with tumors at more advanced FIGO stages, survival rates were influenced by the types of peritoneal implants present: 95.3% for non-invasive peritoneal implants compared with 66% for invasive implants. Neither microinvasion in the primary ovarian tumor nor the presence of BOT cells in the lymph nodes had a significant adverse effect on life expectancy, with survival rates of 100% and 98%, respectively.
Recurrence of borderline tumors
Factors associated with borderline tumor recurrence are: FIGO stage at first diagnosis, incomplete removal of tumor(s) at surgery, and histological subtype (e.g., serous with micropapillary pattern, mucinous tumor of the peritoneum, microinvasion). Recurrent BOT can usually be treated effectively with further surgery, and the 10-year survival rate for recurrent cases is greater than 95%.
Recurrence for BOT can manifest as another BOT, or there can be malignant transformation to LGSC. These are generally slow-growing cancers, and five-year survival rates, even for tumors of advanced FIGO stages, are around 85%.
Typically, non-serous BOTs are FIGO stage 1. Even if there is microinvasion, recurrence or metastases are uncommon. WHO 2014 argues there is little justification in calling these subtypes BOT and instead recommends calling them “atypical proliferative tumors.” (The terms “BOT” and “atypical proliferative tumor” are considered by WHO to be equivalent.)






