The challenges of identifying cardiovascular risk
Despite numerous tools, calculators and algorithms to assess cardiovascular risk, many individuals at apparently low to moderate risk continue to suffer major cardiovascular events. The greatest concern is that a significant proportion of cardiovascular deaths occur in the absence of well-established risk factors such as hypertension, diabetes, obesity, raised lipids or smoking.
At the other end of the spectrum, modestly raised blood pressure is possibly treated unnecessarily as a precaution. New cardiovascular biomarkers such as C-reactive protein, N-terminal pro-atrial natriuretic peptide and homocysteine have only added moderately to standard risk factors for risk assessment1.
Over the past 20 years, arterial stiffness, as measured by aortic pulse wave velocity (aPWV), is being increasingly recognized as a promising biometric to help predict major cardiovascular events and death. The test is likely to be most useful in younger adults with low to moderate cardiovascular risk2. Underwriters will find the aPWV test particularly relevant as a significant proportion of insurance applicants are in this risk group.
Arterial stiffness
Arterial stiffness, also known as hardening of the arteries, is a well-known precursor to cardiovascular disease, including coronary heart disease, stroke and heart failure. Recent evidence suggests arterial stiffness precedes both hypertension and target organ damage. Measurement of arterial stiffness is therefore emerging as a method to predict cardiovascular disease.
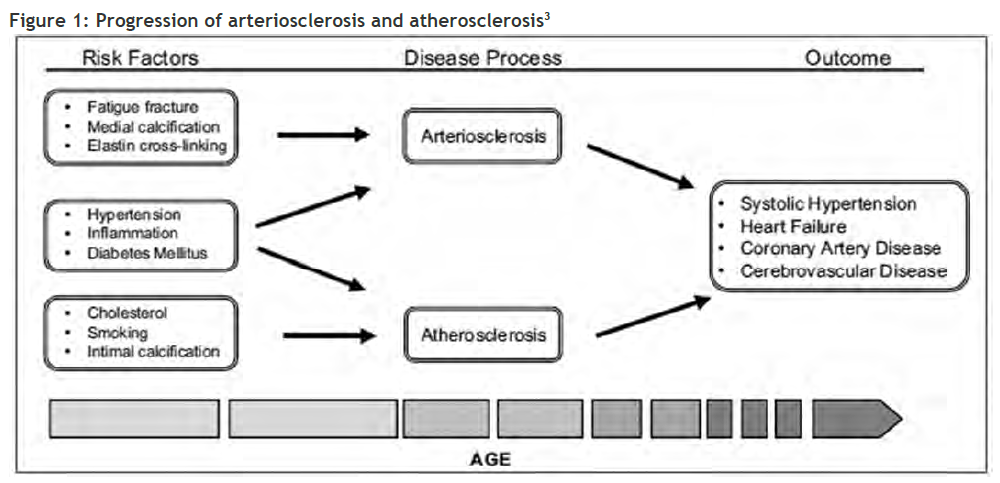
Arteriosclerosis and atherosclerosis are separate pathological entities, driven largely by different mechanisms (Figure 1, page 51). Measuring arterial stiffness is primarily focused on arteriosclerosis rather than atherosclerosis. Arteriosclerosis involves loss of elasticity and dilatation of the large arteries, while atherosclerosis (a form of arteriosclerosis) is characterised by patchy thickening of the inner lining of the artery walls.
Direct measurement of arterial stiffness is technically challenging, requires invasive investigations and is expensive. Instead, aPWV can be used to estimate arterial stiffness and is being increasingly recognised as a reliable technique. Using a complex mathematical formula relating to large-artery haemodynamics, aPWV can be estimated using relatively cheap, noninvasive techniques.
What is pulse wave velocity?
With each heartbeat, a wave, or pulse, travels along arterial vessels. PWV is the speed at which this arterial pulse transmits through the circulatory system. The velocity of this pulse wave is related to the stiffness of the arteries; a higher PWV corresponds to lower arterial distensibility and compliance. In other words, the pulse wave travels faster if the arteries are stiffer.
Carotid-femoral PWV (cfPWV) is regarded as the gold standard measurement methodology, as it includes the aorta and other large arteries that are at highest risk of stiffening. cfPWV is usually measured by placing a non-invasive pressure sensor over the carotid and femoral arteries and then calculating the time difference in the arrival of the pulse (expressed in metres per second). However, measuring cfPWV is not always practical as it requires special equipment and trained technicians. Other disadvantages of cfPWV include the need to measure the distance between the carotid and femoral artery, the patient’s need to undress to allow access to the femoral artery, and finding the pulse in the presence of obesity.
Accurate and reproducible techniques to measure aPWV
Newer techniques to measure aPWV have become available in recent years. Devices that use a cuff around the brachial artery are quicker, more practical and require minimal training. As the technology advances, the cost of measuring aPWV is likely to decrease, allowing it to be used for insurance underwriting assessment at paramedical examinations. Studies have shown cuff-based device measurements to be accurate and reproducible4 (Figure 2, see pdf). Using the brachial pulse wave to derive aPWV has been validated in low- and high-risk cohorts5,6 and is highly reproducible7.
Aortic PWV can also be measured using sophisticated ultrasonography or MRI-based approaches. However, these are considered too expensive and time-consuming for routine clinical medicine.
In the future it may be possible to estimate aPWV with low-cost optical methods using photoplethysmography, i.e., measuring light absorption through blood. However, these devices are at an early stage of validation. Such novel solutions are showing encouraging results and could allow the measurement of arterial stiffness to become part of routine clinical medicine8.
Strong correlation with cardiovascular events and mortality
Pulse wave velocity is strongly correlated with cardiovascular events and all-cause mortality. In 2007 the European Society of Hypertension defined a cfPWV of over 12 m/s as asymptomatic target organ damage9.
A 2015 scientific statement commissioned by the American Heart Association recognized the importance of arterial stiffness. The statement recommended that it was reasonable to measure arterial stiffness to provide incremental information beyond standard cardiovascular risk factors in the prediction of future cardiovascular events10.
A meta-analysis in 2014 analysed data from over 17,000 participants and found that arterial stiffness, assessed by measurement of aPWV, can predict future cardiovascular events and mortality, even after adjusting for established cardiovascular risk factors11.
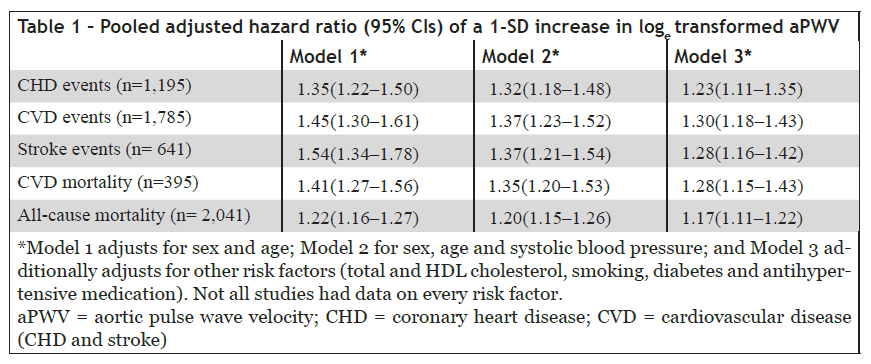
The overall results are summarized in Table 1 and show that aPWV predicts future fatal and non-fatal coronary and stroke events with a hazard ratio ranging from 1.35 to 1.54. Adjustment for established risk factors caused only a modest reduction in predictive value, with a hazard ratio of 1.3. The study also demonstrated that the addition of aPWV to risk prediction models improved risk classification, particularly among younger individuals at intermediate cardiovascular risk.
A 2010 meta-analysis study reviewed data on 15,877 participants12. This study found pooled relative risks of total CV events, CV mortality and all-cause mortality were 2.26 (95% CI 1.89 to 2.70, 14 studies), 2.02 (95% CI 1.68 to 2.42, 10 studies) and 1.90 (95% CI 1.61 to 2.24, 11 studies), respectively, for high vs. low aortic PWV subjects.
AMUS-sponsored research study
In June 2016, the AMUS sponsored a major study in collaboration with Cambridge University Hospitals Trust. AMUS recognized aPWV as a potential way to enable fairer risk selection and stratification of intermediate risk individuals. Therefore the study was initiated with the following hypotheses and aims:
- The addition of aPWV to existing risk calculators improves risk prediction.
- aPWV improves risk prediction in diabetic and pre-diabetic individuals, independent of existing risk factors.
- Dysglycemia is associated with accelerated vascular ageing as assessed by aPWV.
- A cuff-based system provides accurate estimates of aPWV across a range of cardiovascular risk.
The study aimed to test these hypotheses in a series of three sub-studies, summarized as follows:
1. Risk prediction algorithms
Using published meta-analysis data, determine whether adding aPWV to the existing QRISK2 cardiovascular disease risk calculator improves risk prediction. Additionally develop a novel risk calculator using the data from the meta-analysis incorporating aPWV. Separately, validate the usefulness of each of the algorithms using an independent set of data drawn from the Whitehall II study of British civil servants.
2. Diabetes, dysglycemia and arterial stiffness
Using data from the Whitehall II study community=based cohort (n~10,000), determine:
a) If aPWV is elevated in diabetics and pre-diabetics.
b) If there is a continuous relationship between dysglycemia and aPWV.
c) If diabetes, or indices of glycemia, are associated with accelerated progression of aPWV.
d) If measuring aPWV improves our ability to predict future cardiovascular events or of complications of diabetes.
3. Validation of a cuff-based system for aPWV measurement
Confirm the equivalence of aPWV and cfPWV measurement technologies by making duplicate measurements in a large (n=280) cohort of randomly selected individuals.
Also, these data, and data from the first two studies, will be used to undertake a preliminary cost-effective analysis of measuring aPWV in a clinical setting.
Results from all three studies will be published in late 2017 and will be made available to the international underwriting community. We believe these studies offer the potential to make significant improvements in risk-selection, particularly for younger lives who are currently assessed as moderate risks. For example, incorporating aPWV might allow just those at greater cardiovascular risk to be rated.
Conclusion
Pulse wave velocity is becoming a powerful new way to stratify cardiovascular risk and to measure target organ damage in hypertension. The aPWV test is particularly relevant for insurance underwriting assessment, as it is likely to be most useful in assessing a large proportion of applicants, i.e., younger adults with low to moderate cardiovascular risk. Findings from the AMUS-sponsored research studies will allow underwriters to understand how aPWV can be used to enable fairer and more accurate stratification of cardiovascular risk.